Page 79 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 79
2.3 Gas–Liquid Interfacial Behavior 53
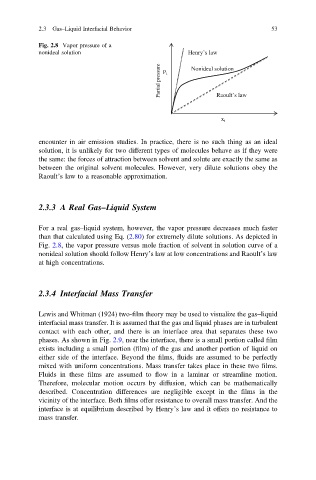
Fig. 2.8 Vapor pressure of a
nonideal solution Henry’s law
Partial pressure P i Nonideal solution
Raoult’s law
x i
encounter in air emission studies. In practice, there is no such thing as an ideal
solution, it is unlikely for two different types of molecules behave as if they were
the same: the forces of attraction between solvent and solute are exactly the same as
between the original solvent molecules. However, very dilute solutions obey the
Raoult’s law to a reasonable approximation.
2.3.3 A Real Gas–Liquid System
For a real gas–liquid system, however, the vapor pressure decreases much faster
than that calculated using Eq. (2.80) for extremely dilute solutions. As depicted in
Fig. 2.8, the vapor pressure versus mole fraction of solvent in solution curve of a
nonideal solution should follow Henry’s law at low concentrations and Raoult’s law
at high concentrations.
2.3.4 Interfacial Mass Transfer
Lewis and Whitman (1924) two-film theory may be used to visualize the gas–liquid
interfacial mass transfer. It is assumed that the gas and liquid phases are in turbulent
contact with each other, and there is an interface area that separates these two
phases. As shown in Fig. 2.9, near the interface, there is a small portion called film
exists including a small portion (film) of the gas and another portion of liquid on
either side of the interface. Beyond the films, fluids are assumed to be perfectly
mixed with uniform concentrations. Mass transfer takes place in these two films.
Fluids in these films are assumed to flow in a laminar or streamline motion.
Therefore, molecular motion occurs by diffusion, which can be mathematically
described. Concentration differences are negligible except in the films in the
vicinity of the interface. Both films offer resistance to overall mass transfer. And the
interface is at equilibrium described by Henry’s law and it offers no resistance to
mass transfer.