Page 77 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 77
2.3 Gas–Liquid Interfacial Behavior 51
Table 2.4 Henry’s law constants for gases in water at different temperatures (H ¼ p i =x i , atm/(mol
gas/mol water))
Gas 0 °C 10 °C 20 °C 30 °C 40 °C 50 °C
He 129,000 126,000 125,000 124,000 121,000 115,000
H 2 57,900 63,600 68,300 72,900 75,100 76,500
52,900 66,800 80,400 92,400 104,000 113,000
N 2
CO 35,200 44,200 53,600 62,000 69,600 76,100
O 2 25,500 32,700 40,100 47,500 53,500 58,800
22,400 29,700 37,600 44,900 52,000 57,700
CH 4
12,600 18,900 26,300 34,200 42,300 50,000
C 2 H 6
C 2 H 4 5,520 7,680 10,200 12,700 – –
728 1,040 1,420 1,860 2,330 2,830
CO 2
H 2 S 268 367 483 609 745 884
The Henry’s law indicates that the equilibrium mole fraction of a gas in liquid is
proportional to the partial pressure of the gas above the liquid regardless of the total
pressure. Generally, this linear relationship (Henry’s law) is sufficiently accurate for
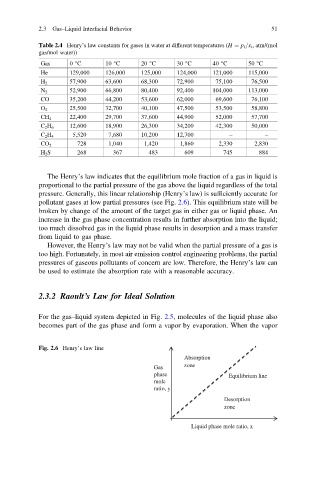
pollutant gases at low partial pressures (see Fig. 2.6). This equilibrium state will be
broken by change of the amount of the target gas in either gas or liquid phase. An
increase in the gas phase concentration results in further absorption into the liquid;
too much dissolved gas in the liquid phase results in desorption and a mass transfer
from liquid to gas phase.
However, the Henry’s law may not be valid when the partial pressure of a gas is
too high. Fortunately, in most air emission control engineering problems, the partial
pressures of gaseous pollutants of concern are low. Therefore, the Henry’s law can
be used to estimate the absorption rate with a reasonable accuracy.
2.3.2 Raoult’s Law for Ideal Solution
For the gas–liquid system depicted in Fig. 2.5, molecules of the liquid phase also
becomes part of the gas phase and form a vapor by evaporation. When the vapor
Fig. 2.6 Henry’s law line
Absorption
Gas zone
phase Equilibrium line
mole
ratio, y
Desorption
zone
Liquid phase mole ratio, x