Page 80 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 80
54 2 Basic Properties of Gases
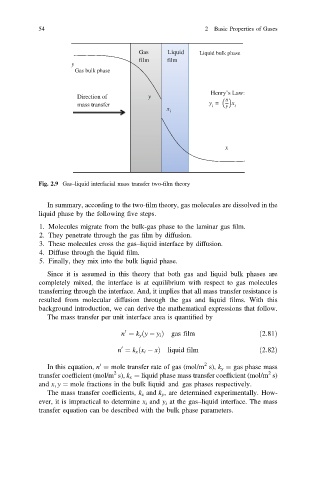
Gas Liquid Liquid bulk phase
film film
y
Gas bulk phase
Henry’s Law:
Direction of y
mass transfer y = x i
i
x
i
x
Fig. 2.9 Gas–liquid interfacial mass transfer two-film theory
In summary, according to the two-film theory, gas molecules are dissolved in the
liquid phase by the following five steps.
1. Molecules migrate from the bulk-gas phase to the laminar gas film.
2. They penetrate through the gas film by diffusion.
3. These molecules cross the gas–liquid interface by diffusion.
4. Diffuse through the liquid film.
5. Finally, they mix into the bulk liquid phase.
Since it is assumed in this theory that both gas and liquid bulk phases are
completely mixed, the interface is at equilibrium with respect to gas molecules
transferring through the interface. And, it implies that all mass transfer resistance is
resulted from molecular diffusion through the gas and liquid films. With this
background introduction, we can derive the mathematical expressions that follow.
The mass transfer per unit interface area is quantified by
0
n ¼ k y y y i Þ gas film ð2:81Þ
ð
0
n ¼ k x x i xð Þ liquid film ð2:82Þ
2
0
In this equation, n ¼ mole transfer rate of gas (mol/m s), k y ¼ gas phase mass
2
2
transfer coefficient (mol/m s), k x ¼ liquid phase mass transfer coefficient (mol/m s)
and x; y ¼ mole fractions in the bulk liquid and gas phases respectively:
The mass transfer coefficients, k x and k y , are determined experimentally. How-
ever, it is impractical to determine x i and y i at the gas–liquid interface. The mass
transfer equation can be described with the bulk phase parameters.