Page 247 - An Introduction to Microelectromechanical Systems Engineering
P. 247
226 Packaging and Reliability Considerations for MEMS
embedded with silver and resin and are mostly used in the brazing of pressed ceramic
packages (e.g., CERDIP type and CERQUAD type) in the integrated circuits
industry. Their utility for die-attach may be limited because of the high-temperature
(400ºC) glass seal and cure operation.
The choice of a solder alloy depends on it having a suitable melting temperature
as well as appropriate mechanical properties. A solder firmly attaches the die to the
package and normally provides little or no stress isolation when compared to
organic adhesives. The large mismatch in the coefficients of thermal expansion with
silicon or glass results in undesirable stresses that can cause cracks in the bond.
However, the bond is very robust and can sustain large normal pull forces on the
2
order of 5,000 N/cm .
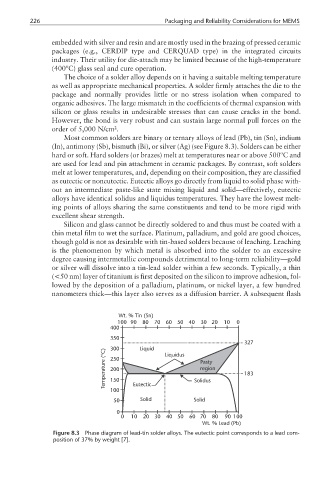
Most common solders are binary or ternary alloys of lead (Pb), tin (Sn), indium
(In), antimony (Sb), bismuth (Bi), or silver (Ag) (see Figure 8.3). Solders can be either
hard or soft. Hard solders (or brazes) melt at temperatures near or above 500ºC and
are used for lead and pin attachment in ceramic packages. By contrast, soft solders
melt at lower temperatures, and, depending on their composition, they are classified
as eutectic or noneutectic. Eutectic alloys go directly from liquid to solid phase with-
out an intermediate paste-like state mixing liquid and solid—effectively, eutectic
alloys have identical solidus and liquidus temperatures. They have the lowest melt-
ing points of alloys sharing the same constituents and tend to be more rigid with
excellent shear strength.
Silicon and glass cannot be directly soldered to and thus must be coated with a
thin metal film to wet the surface. Platinum, palladium, and gold are good choices,
though gold is not as desirable with tin-based solders because of leaching. Leaching
is the phenomenon by which metal is absorbed into the solder to an excessive
degree causing intermetallic compounds detrimental to long-term reliability—gold
or silver will dissolve into a tin-lead solder within a few seconds. Typically, a thin
(<50 nm) layer of titanium is first deposited on the silicon to improve adhesion, fol-
lowed by the deposition of a palladium, platinum, or nickel layer, a few hundred
nanometers thick—this layer also serves as a diffusion barrier. A subsequent flash
Wt. % Tin (Sn)
100 90 80 70 60 50 40 30 20 10 0
400
350
327
(°C) 300 Liquid Liquidus Pasty
250
Temperature 200 Solidus 183
region
150
100 Eutectic
50 Solid Solid
0
0 10 20 30 40 50 60 70 80 90 100
Wt. % Lead (Pb)
Figure 8.3 Phase diagram of lead-tin solder alloys. The eutectic point corresponds to a lead com-
position of 37% by weight [7].