Page 48 - An Introduction to Microelectromechanical Systems Engineering
P. 48
Important Material Properties and Physical Effects 27
to an externally applied voltage. The effect was discovered in quartz by the brothers
Pierre and Jacques Curie in 1880 [22]. Its first practical application was in the
1920s when Langevin developed a quartz transmitter and receiver for underwater
sound—the first Sonar! Piezoelectric crystals are common in many modern applica-
tions (e.g., as clock oscillators in computers and as ringers in cellular telephones).
They are attractive for MEMS because they can be used as sensors as well as actua-
tors, and they can be deposited as thin films over standard silicon substrates.
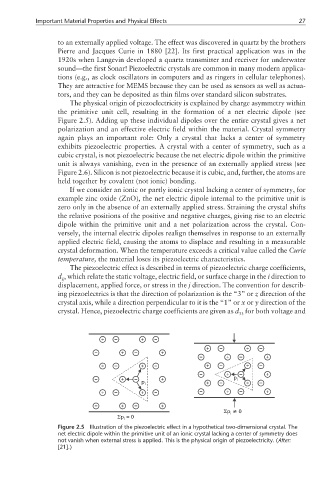
The physical origin of piezoelectricity is explained by charge asymmetry within
the primitive unit cell, resulting in the formation of a net electric dipole (see
Figure 2.5). Adding up these individual dipoles over the entire crystal gives a net
polarization and an effective electric field within the material. Crystal symmetry
again plays an important role: Only a crystal that lacks a center of symmetry
exhibits piezoelectric properties. A crystal with a center of symmetry, such as a
cubic crystal, is not piezoelectric because the net electric dipole within the primitive
unit is always vanishing, even in the presence of an externally applied stress (see
Figure 2.6). Silicon is not piezoelectric because it is cubic, and, further, the atoms are
held together by covalent (not ionic) bonding.
If we consider an ionic or partly ionic crystal lacking a center of symmetry, for
example zinc oxide (ZnO), the net electric dipole internal to the primitive unit is
zero only in the absence of an externally applied stress. Straining the crystal shifts
the relative positions of the positive and negative charges, giving rise to an electric
dipole within the primitive unit and a net polarization across the crystal. Con-
versely, the internal electric dipoles realign themselves in response to an externally
applied electric field, causing the atoms to displace and resulting in a measurable
crystal deformation. When the temperature exceeds a critical value called the Curie
temperature, the material loses its piezoelectric characteristics.
The piezoelectric effect is described in terms of piezoelectric charge coefficients,
d , which relate the static voltage, electric field, or surface charge in the i direction to
ij
displacement, applied force, or stress in the j direction. The convention for describ-
ing piezoelectrics is that the direction of polarization is the “3” or z direction of the
crystal axis, while a direction perpendicular to it is the “1” or x or y direction of the
crystal. Hence, piezoelectric charge coefficients are given as d for both voltage and
33
p
p i
i
Σp i ≠ 0
Σp= 0
i
Figure 2.5 Illustration of the piezoelectric effect in a hypothetical two-dimensional crystal. The
net electric dipole within the primitive unit of an ionic crystal lacking a center of symmetry does
not vanish when external stress is applied. This is the physical origin of piezoelectricity. (After:
[21].)