Page 28 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 28
1-2 FARADAIC PROCESSES 13
the overall current is given by the difference between the currents due to the forward
and backward reactions:
i net i i nFAk C
0; t k C
0; t
1-23
f
O
b
R
f
b
By substituting the expressions for k and k (equations 1-17 and 1-18), one obtains
f
b
i nFAk fC
0; t exp anF
E E =RT
O
C
0; t exp
1 anF
E E =RTg
1-24
R
which describes the current±potential relationship for reactions controlled by the rate
of electron transfer. Note that the net current depends on both the operating potential
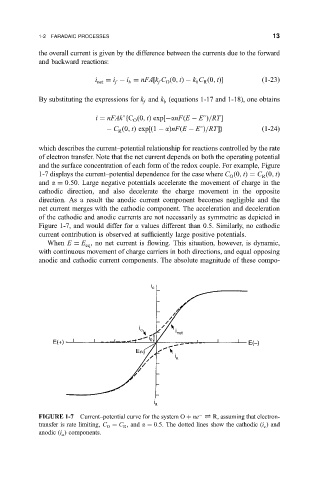
and the surface concentration of each form of the redox couple. For example, Figure
1-7 displays the current±potential dependence for the case where C
0; t C
0; t
R
O
and a 0:50. Large negative potentials accelerate the movement of charge in the
cathodic direction, and also decelerate the charge movement in the opposite
direction. As a result the anodic current component becomes negligible and the
net current merges with the cathodic component. The acceleration and deceleration
of the cathodic and anodic currents are not necessarily as symmetric as depicted in
Figure 1-7, and would differ for a values different than 0.5. Similarly, no cathodic
current contribution is observed at suf®ciently large positive potentials.
When E E , no net current is ¯owing. This situation, however, is dynamic,
eq
with continuous movement of charge carriers in both directions, and equal opposing
anodic and cathodic current components. The absolute magnitude of these compo-
FIGURE 1-7 Current±potential curve for the system O ne R, assuming that electron-
transfer is rate limiting, C C , and a 0:5. The dotted lines show the cathodic (i ) and
c
O
R
anodic (i ) components.
a