Page 31 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 31
16 FUNDAMENTAL CONCEPTS
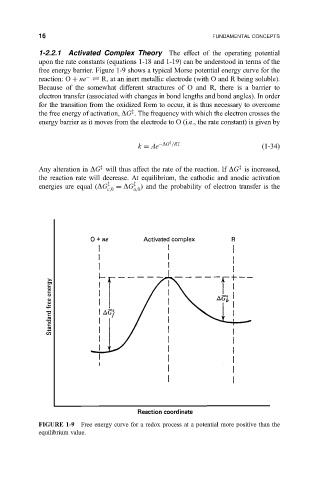
1-2.2.1 Activated Complex Theory The effect of the operating potential
upon the rate constants (equations 1-18 and 1-19) can be understood in terms of the
free energy barrier. Figure 1-9 shows a typical Morse potential energy curve for the
reaction: O ne R, at an inert metallic electrode (with O and R being soluble).
Because of the somewhat different structures of O and R, there is a barrier to
electron transfer (associated with changes in bond lengths and bond angles). In order
for the transition from the oxidized form to occur, it is thus necessary to overcome
the free energy of activation, DG . The frequency with which the electron crosses the
z
energy barrier as it moves from the electrode to O (i.e., the rate constant) is given by
z
k Ae DG =RT
1-34
z
Any alteration in DG will thus affect the rate of the reaction. If DG is increased,
z
the reaction rate will decrease. At equilibrium, the cathodic and anodic activation
z
energies are equal (DG z DG ) and the probability of electron transfer is the
c;0 a;0
FIGURE 1-9 Free energy curve for a redox process at a potential more positive than the
equilibrium value.