Page 30 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 30
1-2 FARADAIC PROCESSES 15
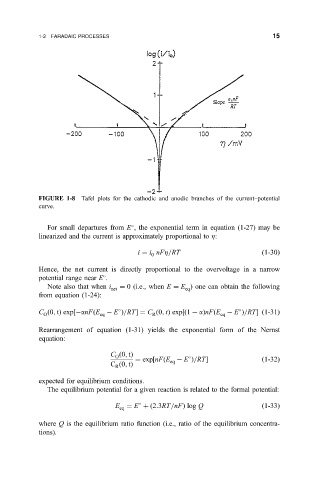
FIGURE 1-8 Tafel plots for the cathodic and anodic branches of the current±potential
curve.
For small departures from E , the exponential term in equation (1-27) may be
linearized and the current is approximately proportional to Z:
i i nFZ=RT
1-30
0
Hence, the net current is directly proportional to the overvoltage in a narrow
potential range near E .
Note also that when i net 0(i.e., when E E ) one can obtain the following
eq
from equation (1-24):
C
0; t exp anF
E E =RT C
0; t exp
1 anF
E E =RT
1-31
eq
R
O
eq
Rearrangement of equation (1-31) yields the exponential form of the Nernst
equation:
C
0; t
O
expnF
E E =RT
1-32
eq
C
0; t
R
expected for equilibrium conditions.
The equilibrium potential for a given reaction is related to the formal potential:
E E
2:3RT=nF log Q
1-33
eq
where Q is the equilibrium ratio function (i.e., ratio of the equilibrium concentra-
tions).