Page 34 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 34
1-3 THE ELECTRICAL DOUBLE LAYER 19
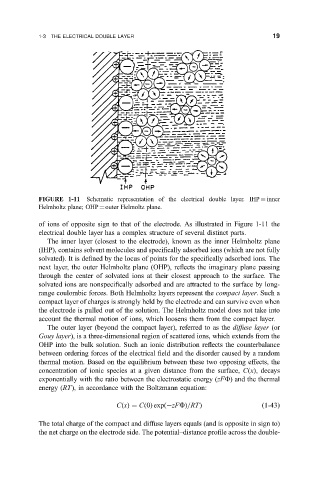
FIGURE 1-11 Schematic representation of the electrical double layer. IHP inner
Helmholtz plane; OHP outer Helmoltz plane.
of ions of opposite sign to that of the electrode. As illustrated in Figure 1-11 the
electrical double layer has a complex structure of several distinct parts.
The inner layer (closest to the electrode), known as the inner Helmholtz plane
(IHP), contains solvent molecules and speci®cally adsorbed ions (which are not fully
solvated). It is de®ned by the locus of points for the speci®cally adsorbed ions. The
next layer, the outer Helmholtz plane (OHP), re¯ects the imaginary plane passing
through the center of solvated ions at their closest approach to the surface. The
solvated ions are nonspeci®cally adsorbed and are attracted to the surface by long-
range coulombic forces. Both Helmholtz layers represent the compact layer. Such a
compact layer of charges is strongly held by the electrode and can survive even when
the electrode is pulled out of the solution. The Helmholtz model does not take into
account the thermal motion of ions, which loosens them from the compact layer.
The outer layer (beyond the compact layer), referred to as the diffuse layer (or
Gouy layer), is a three-dimensional region of scattered ions, which extends from the
OHP into the bulk solution. Such an ionic distribution re¯ects the counterbalance
between ordering forces of the electrical ®eld and the disorder caused by a random
thermal motion. Based on the equilibrium between these two opposing effects, the
concentration of ionic species at a given distance from the surface, C
x, decays
exponentially with the ratio between the electrostatic energy (zFF) and the thermal
energy (RT), in accordance with the Boltzmann equation:
C
x C
0 exp
zFF=RT
1-43
The total charge of the compact and diffuse layers equals (and is opposite in sign to)
the net charge on the electrode side. The potential±distance pro®le across the double-