Page 65 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 65
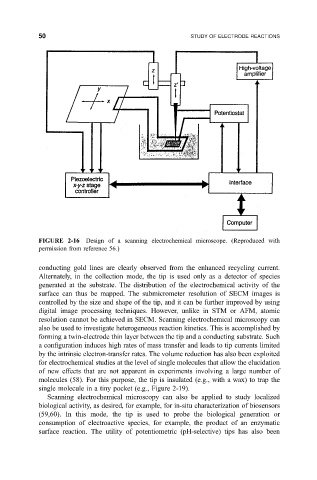
50 STUDY OF ELECTRODE REACTIONS
FIGURE 2-16 Design of a scanning electrochemical microscope. (Reproduced with
permission from reference 56.)
conducting gold lines are clearly observed from the enhanced recycling current.
Alternately, in the collection mode, the tip is used only as a detector of species
generated at the substrate. The distribution of the electrochemical activity of the
surface can thus be mapped. The submicrometer resolution of SECM images is
controlled by the size and shape of the tip, and it can be further improved by using
digital image processing techniques. However, unlike in STM or AFM, atomic
resolution cannot be achieved in SECM. Scanning electrochemical microscopy can
also be used to investigate heterogeneous reaction kinetics. This is accomplished by
forming a twin-electrode thin layer between the tip and a conducting substrate. Such
a con®guration induces high rates of mass transfer and leads to tip currents limited
by the intrinsic electron-transfer rates. The volume reduction has also been exploited
for electrochemical studies at the level of single molecules that allow the elucidation
of new effects that are not apparent in experiments involving a large number of
molecules (58). For this purpose, the tip is insulated (e.g., with a wax) to trap the
single molecule in a tiny pocket (e.g., Figure 2-19).
Scanning electrochemical microscopy can also be applied to study localized
biological activity, as desired, for example, for in-situ characterization of biosensors
(59,60). In this mode, the tip is used to probe the biological generation or
consumption of electroactive species, for example, the product of an enzymatic
surface reaction. The utility of potentiometric (pH-selective) tips has also been