Page 69 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 69
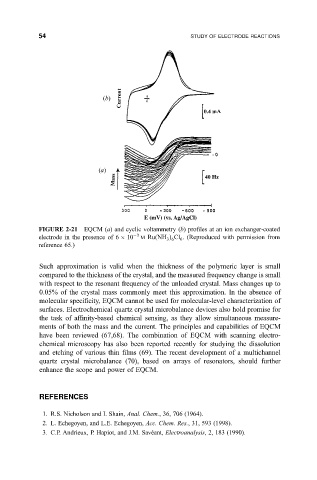
54 STUDY OF ELECTRODE REACTIONS
(b)
(a)
FIGURE 2-21 EQCM (a) and cyclic voltammetry (b) pro®les at an ion exchanger-coated
electrode in the presence of 6 10 3 M Ru
NH Cl . (Reproduced with permission from
6
3 6
reference 65.)
Such approximation is valid when the thickness of the polymeric layer is small
compared to the thickness of the crystal, and the measured frequency change is small
with respect to the resonant frequency of the unloaded crystal. Mass changes up to
0.05% of the crystal mass commonly meet this approximation. In the absence of
molecular speci®city, EQCM cannot be used for molecular-level characterization of
surfaces. Electrochemical quartz crystal microbalance devices also hold promise for
the task of af®nity-based chemical sensing, as they allow simultaneous measure-
ments of both the mass and the current. The principles and capabilities of EQCM
have been reviewed (67,68). The combination of EQCM with scanning electro-
chemical microscopy has also been reported recently for studying the dissolution
and etching of various thin ®lms (69). The recent development of a multichannel
quartz crystal microbalance (70), based on arrays of resonators, should further
enhance the scope and power of EQCM.
REFERENCES
1. R.S. Nicholson and I. Shain, Anal. Chem., 36, 706 (1964).
2. L. Echegoyen, and L.E. Echegoyen, Acc. Chem. Res., 31, 593 (1998).
3. C.P. Andrieux, P. Hapiot, and J.M. Save Âant, Electroanalysis, 2, 183 (1990).