Page 68 - Analytical Electrochemistry 2d Ed - Jospeh Wang
P. 68
2-4 ELECTROCHEMICAL QUARTZ CRYSTAL MICROBALANCE 53
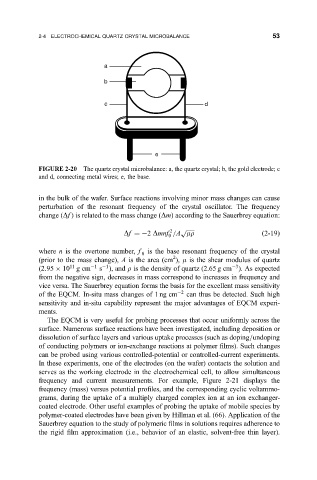
a
b
c d
e
FIGURE 2-20 The quartz crystal microbalance: a, the quartz crystal; b, the gold electrode; c
and d, connecting metal wires; e, the base.
in the bulk of the wafer. Surface reactions involving minor mass changes can cause
perturbation of the resonant frequency of the crystal oscillator. The frequency
change
Df is related to the mass change
Dm according to the Sauerbrey equation:
p
2
Df 2 Dmnf =A mr
2-19
0
where n is the overtone number, f 0 is the base resonant frequency of the crystal
2
(prior to the mass change), A is the area (cm ), m is the shear modulus of quartz
3
1
11
(2:95 10 gcm 1 s , and r is the density of quartz (2.65 g cm ). As expected
from the negative sign, decreases in mass correspond to increases in frequency and
vice versa. The Sauerbrey equation forms the basis for the excellent mass sensitivity
of the EQCM. In-situ mass changes of 1 ng cm 2 can thus be detected. Such high
sensitivity and in-situ capability represent the major advantages of EQCM experi-
ments.
The EQCM is very useful for probing processes that occur uniformly across the
surface. Numerous surface reactions have been investigated, including deposition or
dissolution of surface layers and various uptake processes (such as doping=undoping
of conducting polymers or ion-exchange reactions at polymer ®lms). Such changes
can be probed using various controlled-potential or controlled-current experiments.
In these experiments, one of the electrodes (on the wafer) contacts the solution and
serves as the working electrode in the electrochemical cell, to allow simultaneous
frequency and current measurements. For example, Figure 2-21 displays the
frequency (mass) versus potential pro®les, and the corresponding cyclic voltammo-
grams, during the uptake of a multiply charged complex ion at an ion exchanger-
coated electrode. Other useful examples of probing the uptake of mobile species by
polymer-coated electrodes have been given by Hillman et al. (66). Application of the
Sauerbrey equation to the study of polymeric ®lms in solutions requires adherence to
the rigid ®lm approximation (i.e., behavior of an elastic, solvent-free thin layer).