Page 27 - Analytical method for food addtives
P. 27
22
18
Diode-array
–0.60V using an accumulation potential
the filled cell measuring the absorbance
pH 9.5 borax–NaOH buffer containing
When the flow cell contains C18 silica
the sunset yellow is transported across
hanging mercury drop electrode at:
mM β-cyclodextrin
nm
increase at 487
of –0.40V
5
mL 19 nm 214 Fused-silica capillary column operated M sodium kV with a buffer of 0.05 at 30 mM mM NaH 2 PO 4 /5 deoxycholate in 5 sodium borate at pH 8.6/acetonitrile (17:3) 20 Measurements were carried out directly using an HMDE (hanging mercury dropping electrode) 21 Measurements were made directly. M NH 4 Cl
mL
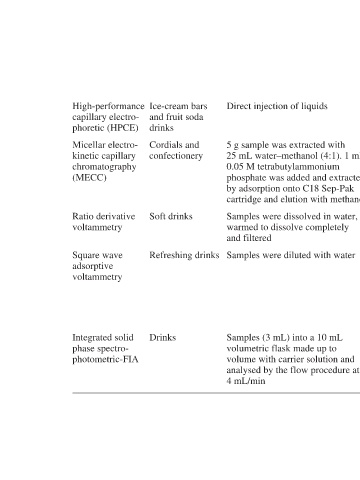
Direct injection of liquids g sample was extracted with 5 mL water–methanol (4:1). 1 25 M tetrabutylammonium 0.05 phosphate was added and extracted by adsorption onto C18 Sep-Pak cartridge and elution with methanol Samples were dissolved in water, warmed to dissolve completely and filtered Samples were diluted with water mL
Ice-cream bars and fruit soda drinks Cordials and confectionery Soft drinks Refreshing drinks Drinks
High-performance capillary electro- phoretic (HPCE) Micellar electro- kinetic capillary chromatography (MECC) Ratio derivative voltammetry Square wave adsorptive voltammetry Integrated solid phase spectro- photometric-FIA