Page 24 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 24
Distillation 13
0.8
* 0.6
-
0
Do
0.4
0.2 -0.4L
-
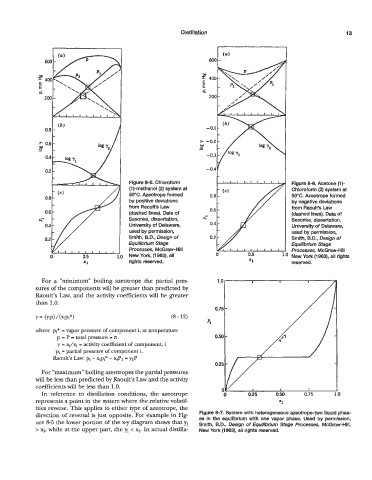
Figure 8-5. Chloroform Figure 8-6. Acetone (1)-
(1)-methanol (2) system at Chloroform (2) system at
50°C. Azeotrope formed 0.8 - 50°C. Azeotrope formed
0.8
by positive deviations by negative deviations
from Raoult’s Law from Raoult’s Law
0.6 (dashed lines). Data of (dashed lines). Data of
h- Sesonke, dissertation, Sesonke, dissertation,
0.4 University of Delaware, University of Delaware,
used by permission, used by permission,
0.2 Smith, B.D., Design of Smith, B.D., Design of
Equilibrium Stage Equilibrium Stage
Processes, McGraw-Hill I I 1 1 1 l 1 1 I. Processes, McGraw-Hill
0 0.5 1 .o New York, (1 963), all 0 0.5 ld New York (1 963), all rights
rights reserved. =I reserved.
For a “minimum” boiling azeotrope the partial pres-
sures of the components will be greater than predicted by
Raoult’s Law, and the activity coefficients will be greater
than 1.0.
Y (YiP)/(xiPi*) (8 - 12)
where pi* = vapor pressure of component i, at temperature
p = P = total pressure = x
y = = activity coefficient of component, i
pi = partial pressure of component i.
Raoult’s Law: pi = xipi* = %PI = YIP
For “maximum” boiling azeotropes the partial pressures
will be less than predicted by Raoult’s Law and the activity
coefficients will be less than 1.0.
In reference to distillation conditions, the azeotrope
represents a point in the system where the relative volatil- =I
ities reverse. This applies to either type of azeotrope, the
direction of reversal is just opposite. For example in Fig- Figure 8-7. System with heterogeneous azeotrope-two liquid phas-
ure 8-5 the lower portion of the x-y diagram shows that yi es in the equilibrium with one vapor phase. Used by permission,
Smith, B.D., Design of Equilibrium Stage Processes, McGraw-Hill,
> xi, while at the upper part, the yi < xi. In actual distilla- New York (1963), all rights reserved.