Page 25 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 25
14 Applied Process Design for Chemical and Petrochemical Plants
M
I
E 500
E
300
I
t- Vaoor
Liquid
50t
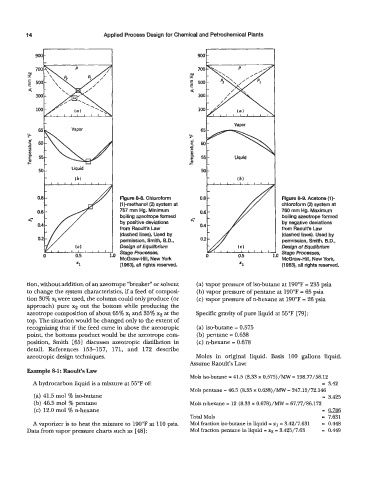
Figure 8-8. Chloroform Figure 8-9. Acetone (1)-
(1)-methanol (2) system at chloroform (2) system at
757 mm Hg. Minimum 760 mm Hg. Maximum
boiling azeotrope formed boiling azeotrope formed
by positive deviations by negative deviations
from Raoult’s Law from Raoult’s Law
(dashed lines). Used by (dashed lines). Used by
permission, Smith, B.D., permission, Smith, B.D.,
Design of Equilibrium Design of Equilibrium
Stage Processes, Stage Processes,
McGraw-Hill, New York McGraw-Hill, New York,
(1963), all rights reserved. (1963), all rights reserved.
tion, without addition of an azeotrope “breaker” or solvent (a) vapor pressure of iso-butane at 190°F = 235 psia
to change the system characteristics, if a feed of composi- (b) vapor pressure of pentane at 190°F = 65 psia
tion 30% 3 were used, the column could only produce (or (c) vapor pressure of n-hexane at 190°F = 26 psia
approach) pure x2 out the bottom while producing the
azeotrope composition of about 65% and 35% x2 at the Specific gravity of pure liquid at 55°F [79] :
top. The situation would be changed only to the extent of
recognizing that if the feed came in above the azeotropic (a) iso-butane = 0.575
point, the bottoms product would be the azeotrope com- (b) pentane = 0.638
position, Smith [631 discusses azeotropic distillation in (c) n-hexane = 0.678
detail. References 153-157, 171, and 172 describe
azeotropic design techniques. Moles in original liquid. Basis 100 gallons liquid.
Assume Raoult’s Law:
Example 8-1: Raoult’s Law
Mols iso-butane = 41.5 (8.33 x 0.575)/MW = 198.77/58.12
A hydrocarbon liquid is a mixture at 55°F of: = 3.42
Mols pentane = 46.5 (8.33 x 0.638)/MW = 247.12/72.146
(a) 41.5 mol % iso-butane = 3.425
(b) 46.5 mol % pentane Mols n-hexane = 12 (8.33 x 0.678)/MW = 67.77/86.172
(c) 12.0 mol % n-hexane =0.786
Total Mols = 7.631
A vaporizer is to heat the mixture to 190°F at 110 psia. Mol fraction iso-butane in liquid = x1 = 3.42/7.631 = 0.448
Data from vapor pressure charts such as [48] : Mol fraction pentane in liquid = x2 = 3.425/7.63 = 0.449