Page 238 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 238
GROUP 16 ELEMENTS: THE CHALCOGENS
218
it makes the image permanent and light resistant. In the age of digital photography, this
once-crucial process is increasingly of historical interest only.
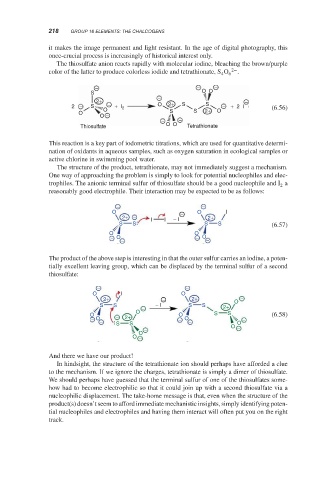
The thiosulfate anion reacts rapidly with molecular iodine, bleaching the brown/purple
color of the latter to produce colorless iodide and tetrathionate, S O 6 2− .
4
− − −
S O O
2+ − − −
2 − S − + I 2 O 2+ S S − + 2 I
O S S 2+ O (6.56)
O O O −
− −
Thiosulfate O O Tetrathionate
This reaction is a key part of iodometric titrations, which are used for quantitative determi-
nation of oxidants in aqueous samples, such as oxygen saturation in ecological samples or
active chlorine in swimming pool water.
The structure of the product, tetrathionate, may not immediately suggest a mechanism.
One way of approaching the problem is simply to look for potential nucleophiles and elec-
trophiles. The anionic terminal sulfur of thiosulfate should be a good nucleophile and I a
2
reasonably good electrophile. Their interaction may be expected to be as follows:
− −
O − O I
2+ − I I − I 2+
S S S S (6.57)
O O
− O − − O −
The product of the above step is interesting in that the outer sulfur carries an iodine, a poten-
tially excellent leaving group, which can be displaced by the terminal sulfur of a second
thiosulfate:
− −
O I O
2+ − 2+ O −
S S − − I S S 2+
O
O − O S S (6.58)
− O − S 2+ S − O − O −
− O −
O
O −
And there we have our product!
In hindsight, the structure of the tetrathionate ion should perhaps have afforded a clue
to the mechanism. If we ignore the charges, tetrathionate is simply a dimer of thiosulfate.
We should perhaps have guessed that the terminal sulfur of one of the thiosulfates some-
how had to become electrophilic so that it could join up with a second thiosulfate via a
nucleophilic displacement. The take-home message is that, even when the structure of the
product(s) doesn’t seem to afford immediate mechanistic insights, simply identifying poten-
tial nucleophiles and electrophiles and having them interact will often put you on the right
track.