Page 239 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 239
6.7 SULFUR OXOCHLORIDES 219
6.7 SULFUR OXOCHLORIDES
Sulfur forms two well-known oxochlorides—thionyl chloride (SOCl ) and sulfuryl chlo-
2
ride (SO Cl ). Both are important reagents in organic chemistry and inorganic chemistry.
2 2
For reasons of space, we will focus here largely on SOCl . As far as SO Cl is concerned,
2 2 2
we will note that it is prepared from SO and Cl in the presence of a catalyst such as
2 2
activated charcoal:
SO + Cl → SO Cl (6.59)
2 2 2 2
In Section 6.15, we will encounter SO Cl again, in the role of an oxidant in the synthesis
2
2
of the remarkable compound S N .
4
4
The main industrial synthesis of SOCl is based on the reaction of SO and SCl :
3
2
2
SO + SCl → SOCl + SO (6.60)
3 2 2 2
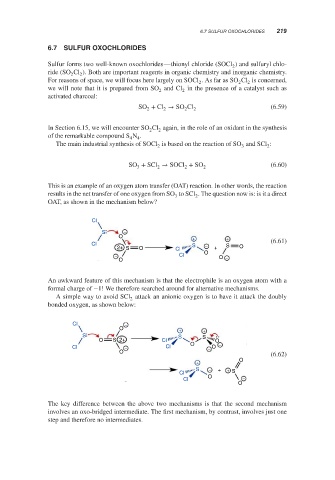
This is an example of an oxygen atom transfer (OAT) reaction. In other words, the reaction
results in the net transfer of one oxygen from SO to SCl . The question now is: is it a direct
3
2
OAT, as shown in the mechanism below?
Cl
S −
O
+ +
Cl S S (6.61)
2+ S O Cl − + O
− Cl O O −
O
An awkward feature of this mechanism is that the electrophile is an oxygen atom with a
formal charge of −1! We therefore searched around for alternative mechanisms.
A simple way to avoid SCl attack an anionic oxygen is to have it attack the doubly
2
bonded oxygen, as shown below:
Cl −
O
+ +
S S S
O S 2+ Cl O
Cl − Cl O − O −
O
(6.62)
O
+
S − + + S
Cl
Cl O −
O
The key difference between the above two mechanisms is that the second mechanism
involves an oxo-bridged intermediate. The first mechanism, by contrast, involves just one
step and therefore no intermediates.