Page 244 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 244
GROUP 16 ELEMENTS: THE CHALCOGENS
224
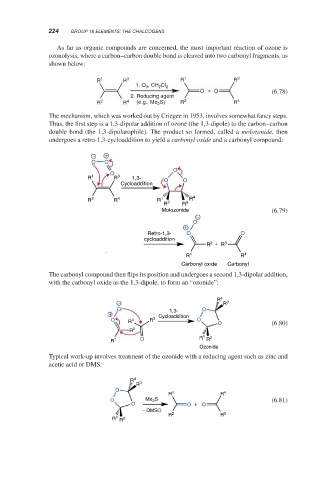
As far as organic compounds are concerned, the most important reaction of ozone is
ozonolysis, where a carbon–carbon double bond is cleaved into two carbonyl fragments, as
shown below:
R 1 R 3 R 1 R 3
1. O , CH Cl 2
3
2
O + O (6.78)
2. Reducing agent
4
R 2 R (e.g., Me 2 S) R 2 R 4
The mechanism, which was worked out by Criegee in 1953, involves somewhat fancy steps.
Thus, the first step is a 1,3-dipolar addition of ozone (the 1,3-dipole) to the carbon–carbon
double bond (the 1,3-dipolarophile). The product so formed, called a molozonide, then
undergoes a retro-1,3-cycloaddition to yield a carbonyl oxide and a carbonyl compound:
− +
O O
O
O
R 1 R 3 1,3- O O
Cycloaddition
R 2 R 4 R 1 R 4
R 2 R 3
Molozonide (6.79)
−
O
+
Retro-1,3- O O
cycloaddition
2
R + R 3
R 1 R 4
Carbonyl oxide Carbonyl
The carbonyl compound then flips its position and undergoes a second 1,3-dipolar addition,
with the carbonyl oxide as the 1,3-dipole, to form an “ozonide”:
− R 4 R 3
O 1,3- O
+ Cycloaddition
O R 4 R 3 O O (6.80)
R 2
1
R 1 O R R 2
Ozonide
Typical work-up involves treatment of the ozonide with a reducing agent such as zinc and
acetic acid or DMS:
R 4
R 3
O
R 1 R 4
O Me 2 S (6.81)
O O + O
− DMSO
R 2 R 3
1
R R 2