Page 247 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 247
6.9 SWERN AND RELATED OXIDATIONS 227
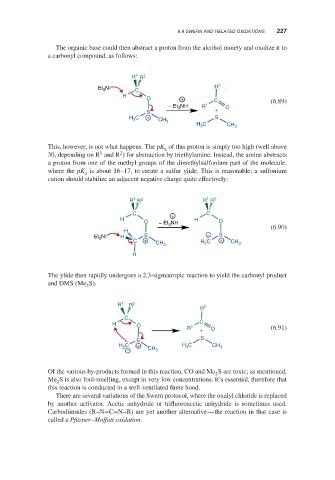
The organic base could then abstract a proton from the alcohol moiety and oxidize it to
a carbonyl compound, as follows:
1
R R 2
Et 3 N C R 2
H
O + C (6.89)
− Et 3 NH R 1 O
S +
C + S
H 3 CH 3
H 3 C CH 3
This, however, is not what happens. The pK of this proton is simply too high (well above
a
1
2
30, depending on R and R ) for abstraction by triethylamine. Instead, the amine abstracts
a proton from one of the methyl groups of the dimethylsulfonium part of the molecule,
where the pK is about 16–17, to create a sulfur ylide. This is reasonable; a sulfonium
a
cation should stabilize an adjacent negative charge quite effectively:
1
1
R R 2 R R 2
C + C
H O − Et 3 NH H O
(6.90)
H
Et N H S − S
3
C + H C +
CH 3 2 CH 3
H
The ylide then rapidly undergoes a 2,3-sigmatropic reaction to yield the carbonyl product
and DMS (Me S).
2
R 1 R 2
R 2
C
H O 1 C
R O (6.91)
+
S S
H 2 C + H 3 C CH 3
− CH 3
Of the various by-products formed in this reaction, CO and Me S are toxic; as mentioned,
2
Me S is also foul-smelling, except in very low concentrations. It’s essential, therefore that
2
this reaction is conducted in a well-ventilated fume hood.
There are several variations of the Swern protocol, where the oxalyl chloride is replaced
by another activator. Acetic anhydride or trifluoroacetic anhydride is sometimes used.
Carbodiimides (R–N=C=N–R) are yet another alternative—the reaction in that case is
called a Pfitzner–Moffatt oxidation.