Page 250 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 250
GROUP 16 ELEMENTS: THE CHALCOGENS
230
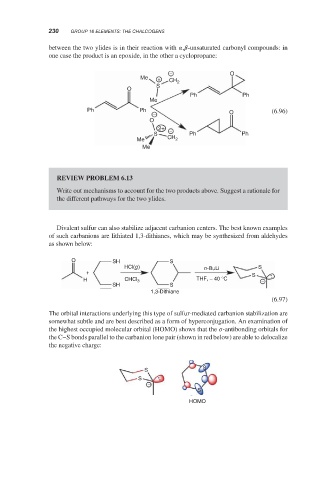
between the two ylides is in their reaction with , -unsaturated carbonyl compounds: in
one case the product is an epoxide, in the other a cyclopropane:
− O
Me + CH
S 2
O
Ph Ph
Me
Ph Ph O (6.96)
−
O
2+ −
S Ph Ph
Me CH 2
Me
REVIEW PROBLEM 6.13
Write out mechanisms to account for the two products above. Suggest a rationale for
the different pathways for the two ylides.
Divalent sulfur can also stabilize adjacent carbanion centers. The best known examples
of such carbanions are lithiated 1,3-dithianes, which may be synthesized from aldehydes
as shown below:
O SH S
HCl(g) n-BuLi S
+
S
H CHCl 3 THF, − 40 °C −
SH S
1,3-Dithiane
(6.97)
The orbital interactions underlying this type of sulfur-mediated carbanion stabilization are
somewhat subtle and are best described as a form of hyperconjugation. An examination of
the highest occupied molecular orbital (HOMO) shows that the -antibonding orbitals for
the C–S bonds parallel to the carbanion lone pair (shown in red below) are able to delocalize
the negative charge:
S
S
S
−
S
HOMO