Page 253 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 253
6.11 HYDROLYSIS OF S F : A MECHANISTIC PUZZLE
2 2 233
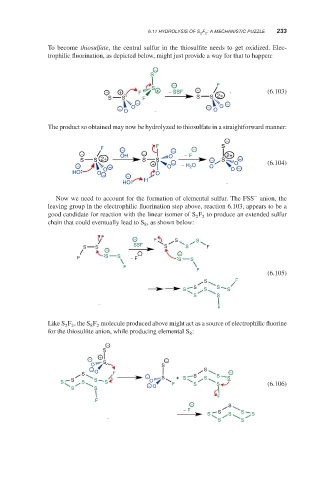
To become thiosulfate, the central sulfur in the thiosulfite needs to get oxidized. Elec-
trophilic fluorination, as depicted below, might just provide a way for that to happen:
−
S
− F
S
− + F + − SSF − (6.103)
S S F S S 2+
− −
− O − O O
O
The product so obtained may now be hydrolyzed to thiosulfate in a straightforward manner:
−
F − F − S
− OH − O − − F 2+
S S 2+ S S − − S −
O
− − + O − H 2 O O O − (6.104)
HO O − O − O
HO H
−
Now we need to account for the formation of elemental sulfur. The FSS anion, the
leaving group in the electrophilic fluorination step above, reaction 6.103, appears to be a
good candidate for reaction with the linear isomer of S F to produce an extended sulfur
2 2
chain that could eventually lead to S , as shown below:
8
F
− F S S
SSF
S S S S F
−
− −
F S S − F S S
F
F
(6.105)
S F
S S
S S S
S S
F
Like S F ,the S F molecule produced above might act as a source of electrophilic fluorine
2 2
8 2
for the thiosulfite anion, while producing elemental S :
8
−
S
− + −
O S S
− S
S O F − S S −
S S S + S S S
S S S O F S S (6.106)
− O
S S
F
F
− S
− F S S
S S S
S S