Page 252 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 252
GROUP 16 ELEMENTS: THE CHALCOGENS
232
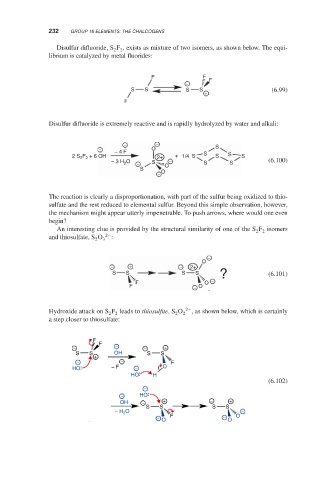
Disulfur difluoride, S F , exists as mixture of two isomers, as shown below. The equi-
2 2
librium is catalyzed by metal fluorides:
F F F
−
S S S S (6.99)
+
F
Disulfur difluoride is extremely reactive and is rapidly hydrolyzed by water and alkali:
− − S
− − 4 F O
2 S F + 6 OH 2+ + 1/4 S S S S S
2 2
− 3 H O − S − S S (6.100)
2
S O O
−
The reaction is clearly a disproportionation, with part of the sulfur being oxidized to thio-
sulfate and the rest reduced to elemental sulfur. Beyond this simple observation, however,
the mechanism might appear utterly impenetrable. To push arrows, where would one even
begin?
An interesting clue is provided by the structural similarity of one of the S F isomers
2 2
2−
and thiosulfate, S O 3 :
2
−
O
− + − 2+
S S S S (6.101)
−
F O
F − O
2−
Hydroxide attack on S F leads to thiosulfite,S O 2 , as shown below, which is certainly
2
2 2
a step closer to thiosulfate:
F
F
− − − +
S S OH S S
+
− − F
HO − F − O
HO H
(6.102)
−
− HO
OH − + − +
S S S S
− H 2 O F O −
− O − O