Page 249 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 249
6.10 SULFUR YLIDES AND SULFUR-STABILIZED CARBANIONS 229
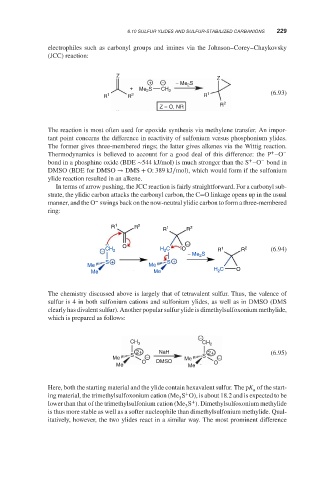
electrophiles such as carbonyl groups and imines via the Johnson–Corey–Chaykovsky
(JCC) reaction:
Z Z
+ − − Me S
2
+ Me S CH 2
2
R 1 R 2 R 1 (6.93)
Z = O, NR R 2
The reaction is most often used for epoxide synthesis via methylene transfer. An impor-
tant point concerns the difference in reactivity of sulfonium versus phosphonium ylides.
The former gives three-membered rings; the latter gives alkenes via the Wittig reaction.
+
Thermodynamics is believed to account for a good deal of this difference: the P –O −
+ −
bond in a phosphine oxide (BDE ∼544 kJ/mol) is much stronger than the S –O bond in
DMSO (BDE for DMSO → DMS + O: 389 kJ∕mol), which would form if the sulfonium
ylide reaction resulted in an alkene.
In terms of arrow pushing, the JCC reaction is fairly straightforward. For a carbonyl sub-
strate, the ylidic carbon attacks the carbonyl carbon, the C=O linkage opens up in the usual
−
manner, and the O swings back on the now-neutral ylidic carbon to form a three-membered
ring:
R 1 R 2 1 2
R R
−
O 1 2
− CH 2 H 2 C O R R (6.94)
− Me 2 S
S + S +
Me Me
H C O
Me Me 2
The chemistry discussed above is largely that of tetravalent sulfur. Thus, the valence of
sulfur is 4 in both sulfonium cations and sulfonium ylides, as well as in DMSO (DMS
clearly has divalent sulfur). Another popular sulfur ylide is dimethylsulfoxonium methylide,
which is prepared as follows:
−
CH 3 CH 2
2+ NaH 2+ (6.95)
S S
Me − Me −
O DMSO O
Me Me
Here, both the starting material and the ylide contain hexavalent sulfur. The pK of the start-
a
+
ing material, the trimethylsulfoxonium cation (Me S O), is about 18.2 and is expected to be
3
+
lower than that of the trimethylsulfonium cation (Me S ). Dimethylsulfoxonium methylide
3
is thus more stable as well as a softer nucleophile than dimethylsulfonium methylide. Qual-
itatively, however, the two ylides react in a similar way. The most prominent difference