Page 246 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 246
GROUP 16 ELEMENTS: THE CHALCOGENS
226
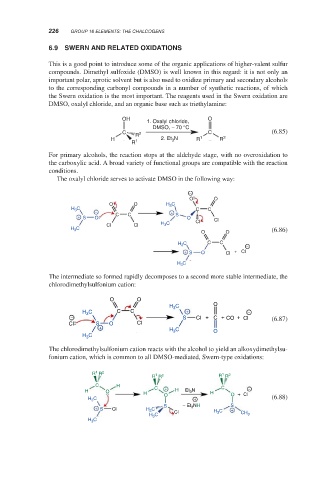
6.9 SWERN AND RELATED OXIDATIONS
This is a good point to introduce some of the organic applications of higher-valent sulfur
compounds. Dimethyl sulfoxide (DMSO) is well known in this regard: it is not only an
important polar, aprotic solvent but is also used to oxidize primary and secondary alcohols
to the corresponding carbonyl compounds in a number of synthetic reactions, of which
the Swern oxidation is the most important. The reagents used in the Swern oxidation are
DMSO, oxalyl chloride, and an organic base such as triethylamine:
OH O
1. Oxalyl chloride,
DMSO, − 70 °C
C 2 C (6.85)
R
H 2. Et N R 1 R 2
3
R 1
For primary alcohols, the reaction stops at the aldehyde stage, with no overoxidation to
the carboxylic acid. A broad variety of functional groups are compatible with the reaction
conditions.
The oxalyl chloride serves to activate DMSO in the following way:
−
O O
O O H 3 C
H 3 C C C
− +
+ S O C C S O Cl
H 3 C Cl
Cl Cl
H 3 C
O O (6.86)
H 3 C C C
−
+ S O Cl + Cl
H 3 C
The intermediate so formed rapidly decomposes to a second more stable intermediate, the
chlorodimethylsulfonium cation:
O O
H 3 C O
C C C +
H 3 −
− S Cl + C + CO + Cl (6.87)
Cl S O Cl
+
H 3 C O
H 3 C
The chlorodimethylsulfonium cation reacts with the alcohol to yield an alkoxydimethylsu-
fonium cation, which is common to all DMSO-mediated, Swern-type oxidations:
1
R R 2 1 1 2
R R 2 R R
C H C
H O H C + H Et 3 N H O + Cl −
H 3 C O + (6.88)
S − Et 3 NH S
+ S Cl H 3 C Cl H 3 C +
H 3 C CH 3
H 3 C