Page 258 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 258
GROUP 16 ELEMENTS: THE CHALCOGENS
238
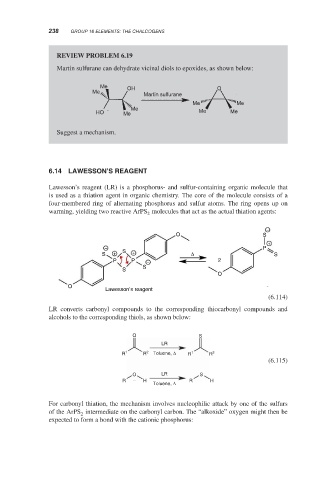
REVIEW PROBLEM 6.19
Martin sulfurane can dehydrate vicinal diols to epoxides, as shown below:
Me OH O
Me
Martin sulfurane
Me Me
Me
HO Me Me Me
Suggest a mechanism.
LAWESSON’S REAGENT
6.14
Lawesson’s reagent (LR) is a phosphorus- and sulfur-containing organic molecule that
is used as a thiation agent in organic chemistry. The core of the molecule consists of a
four-membered ring of alternating phosphorus and sulfur atoms. The ring opens up on
warming, yielding two reactive ArPS molecules that act as the actual thiation agents:
2
−
O S
+
− P
S
S + + Δ S
P P − 2
S
S
O
O
Lawesson’s reagent
(6.114)
LR converts carbonyl compounds to the corresponding thiocarbonyl compounds and
alcohols to the corresponding thiols, as shown below:
O S
LR
R 1 R 2 Toluene, Δ R 1 R 2
(6.115)
O LR S
R H R H
Toluene, Δ
For carbonyl thiation, the mechanism involves nucleophilic attack by one of the sulfurs
of the ArPS intermediate on the carbonyl carbon. The “alkoxide” oxygen might then be
2
expected to form a bond with the cationic phosphorus: