Page 259 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 259
6.14 LAWESSON’S REAGENT 239
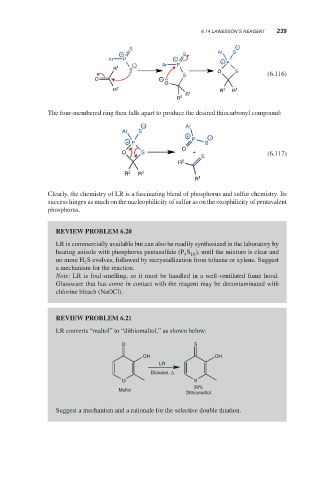
S −
Ar S
+ S
Ar P + +
− Ar P P
R 1 S O S
S (6.116)
O −
O
R 2 R 2 R 1
R 1
R 2
The four-membered ring then falls apart to produce the desired thiocarbonyl compound:
− Ar
Ar S
+ −
+ P P S
O
O S + (6.117)
S
R 2
R 2 R 1
R 1
Clearly, the chemistry of LR is a fascinating blend of phosphorus and sulfur chemistry. Its
success hinges as much on the nucleophilicity of sulfur as on the oxophilicity of pentavalent
phosphorus.
REVIEW PROBLEM 6.20
LR is commercially available but can also be readily synthesized in the laboratory by
heating anisole with phosphorus pentasulfide (P S ), until the mixture is clear and
4 10
no more H S evolves, followed by recrystallization from toluene or xylene. Suggest
2
a mechanism for the reaction.
Note: LR is foul-smelling, so it must be handled in a well-ventilated fume hood.
Glassware that has come in contact with the reagent may be decontaminated with
chlorine bleach (NaOCl).
REVIEW PROBLEM 6.21
LR converts “maltol” to “dithiomaltol,” as shown below:
O S
OH OH
LR
Dioxane, Δ
O S
39%
Maltol
Dithiomaltol
Suggest a mechanism and a rationale for the selective double thiation.