Page 262 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 262
GROUP 16 ELEMENTS: THE CHALCOGENS
242
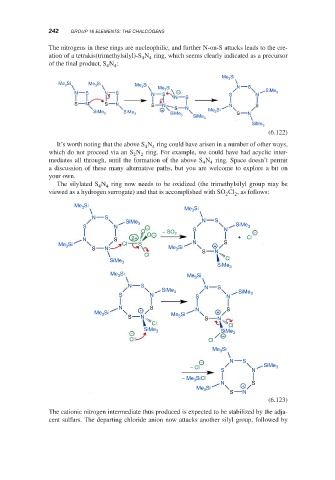
The nitrogens in these rings are nucleophilic, and further N-on-S attacks leads to the cre-
ation of a tetrakis(trimethylsilyl)-S N ring, which seems clearly indicated as a precursor
4
4
of the final product, S N :
4 4
Me 3 Si
Me 3 Si Me 3 Si Me Si Me 3 Si N S
3
N S N S N S − S N SiMe 3
N S
S N S N S N N S
+ S N Me 3 Si
SiMe 3 SiMe 3 SiMe S N
3
SiMe 3
SiMe 3
(6.122)
It’s worth noting that the above S N ring could have arisen in a number of other ways,
4
4
which do not proceed via an S N ring. For example, we could have had acyclic inter-
2 2
mediates all through, until the formation of the above S N ring. Space doesn’t permit
4 4
a discussion of these many alternative paths, but you are welcome to explore a bit on
your own.
The silylated S N ring now needs to be oxidized (the trimethylsilyl group may be
4
4
viewed as a hydrogen surrogate) and that is accomplished with SO Cl , as follows:
2
2
Me 3 Si
Me Si
3
N S
SiMe 3 N S
S N − SiMe 3
O O − − SO 2 S N −
N S 2+ + Cl
Si Cl S N S
Me 3 +
S N Me 3 Si S N
Cl
SiMe 3 Cl
SiMe 3
Me Si Me Si
3
3
N S N S
SiMe 3 SiMe 3
S N S N
N S N S
Me Si + Me Si +
3
S N 3 S N
Cl Cl
− SiMe 3 SiMe 3
−
Cl Cl
Si
Me 3
− N S
− Cl SiMe 3
S N
− Me 3 SiCl
N S
Si +
Me 3
S N
(6.123)
The cationic nitrogen intermediate thus produced is expected to be stabilized by the adja-
cent sulfurs. The departing chloride anion now attacks another silyl group, followed by