Page 265 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 265
6.16 SELENIUM-MEDIATED OXIDATIONS 245
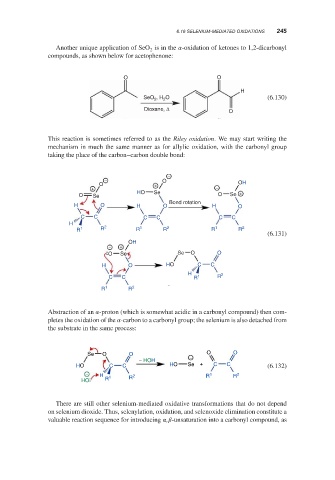
Another unique application of SeO is in the -oxidation of ketones to 1,2-dicarbonyl
2
compounds, as shown below for acetophenone:
O O
H
2
SeO 2 , H O (6.130)
Dioxane, Δ
O
This reaction is sometimes referred to as the Riley oxidation. We may start writing the
mechanism in much the same manner as for allylic oxidation, with the carbonyl group
taking the place of the carbon–carbon double bond:
−
− O
O + OH
+ −
O Se HO Se O Se +
Bond rotation
H O H O H O
C C C C C C
H 2
R 1 R R 1 R 2 R 1 R 2
(6.131)
OH
− +
O Se Se O O
H O HO C C
H 2
C C R 1 R
R 1 R 2
Abstraction of an -proton (which is somewhat acidic in a carbonyl compound) then com-
pletes the oxidation of the -carbon to a carbonyl group; the selenium is also detached from
the substrate in the same process:
Se O O O O
− HOH −
HO C C HO Se + C C (6.132)
− H 2 R 1 R 2
HO R 1 R
There are still other selenium-mediated oxidative transformations that do not depend
on selenium dioxide. Thus, selenylation, oxidation, and selenoxide elimination constitute a
valuable reaction sequence for introducing , -unsaturation into a carbonyl compound, as