Page 266 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 266
GROUP 16 ELEMENTS: THE CHALCOGENS
246
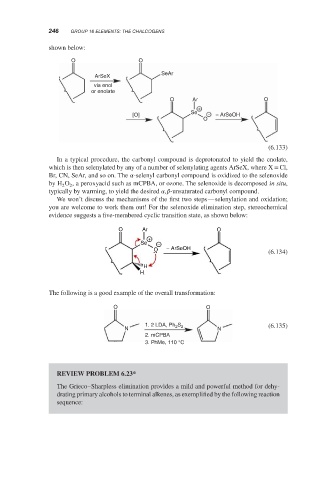
shown below:
O O
SeAr
ArSeX
via enol
or enolate
O Ar O
+
Se
[O] − − ArSeOH
O
(6.133)
In a typical procedure, the carbonyl compound is deprotonated to yield the enolate,
which is then selenylated by any of a number of selenylating agents ArSeX, where X = Cl,
Br, CN, SeAr, and so on. The -selenyl carbonyl compound is oxidized to the selenoxide
by H O , a peroxyacid such as mCPBA, or ozone. The selenoxide is decomposed in situ,
2
2
typically by warming, to yield the desired , -unsaturated carbonyl compound.
We won’t discuss the mechanisms of the first two steps—selenylation and oxidation;
you are welcome to work them out! For the selenoxide elimination step, stereochemical
evidence suggests a five-membered cyclic transition state, as shown below:
O Ar O
+
Se −
O − ArSeOH
(6.134)
H
H
The following is a good example of the overall transformation:
O O
S
1. 2 LDA, Ph 2 2 (6.135)
N N
2. mCPBA
3. PhMe, 110 °C
REVIEW PROBLEM 6.23*
The Grieco–Sharpless elimination provides a mild and powerful method for dehy-
drating primary alcohols to terminal alkenes, as exemplified by the following reaction
sequence: