Page 263 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 263
6.16 SELENIUM-MEDIATED OXIDATIONS 243
elimination of trimethylsilyl chloride:
Me Si Me Si Me 3 Si
3
3
N S N S N S
SiMe SiMe SiMe
3
S N 3 S N − Me SiCl S N 3
3
N S − N S N − S
Me Si + Me 3 Si + +
3
S N S N S N
−
Cl
Cl
(6.124)
The process then continues in a similar manner to produce the final product S N :
4
4
Me Si
3
+
N S N S N S
SiMe 3 + −
S N S − N S N
− − −
N S N S N S
+ +
S N S N S N
+
(6.125)
Despite the complexity of the process, the deliberate choice of sophisticated starting mate-
rials leaves little doubt that the chemists who developed the above synthesis foresaw the
essentials of the mechanism. That is not only a testament to their insight but also a remark-
able demonstration of how mechanistic thinking can guide synthesis design in inorganic
chemistry.
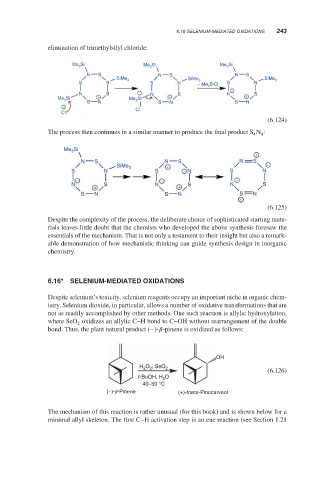
6.16* SELENIUM-MEDIATED OXIDATIONS
Despite selenium’s toxicity, selenium reagents occupy an important niche in organic chem-
istry. Selenium dioxide, in particular, allows a number of oxidative transformations that are
not as readily accomplished by other methods. One such reaction is allylic hydroxylation,
where SeO oxidizes an allylic C–H bond to C–OH without rearrangement of the double
2
bond. Thus, the plant natural product (−)- -pinene is oxidized as follows:
OH
O , SeO
H 2 2 2
(6.126)
t-BuOH, H O
2
40–50 °C
(−)- -Pinene (+)-trans-Pinocarveol
The mechanism of this reaction is rather unusual (for this book) and is shown below for a
minimal allyl skeleton. The first C–H activation step is an ene reaction (see Section 1.21