Page 260 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 260
GROUP 16 ELEMENTS: THE CHALCOGENS
240
6.15 SULFUR NITRIDES
The sulfur nitrides are a fascinating class of main-group compounds. Their structures and
bonding are diverse and subtle; both their formation and their reactions involve stunningly
complicated stoichiometries. These factors tend to discourage in-class discussion of these
compounds, even though many textbooks dutifully describe these remarkable molecules.
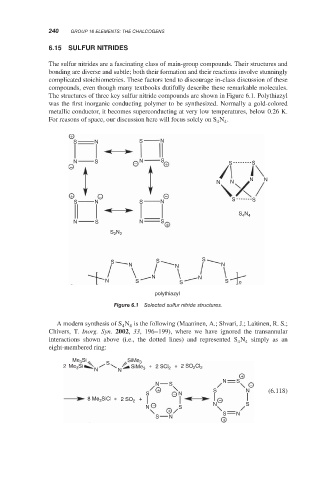
The structures of three key sulfur nitride compounds are shown in Figure 6.1. Polythiazyl
was the first inorganic conducting polymer to be synthesized. Normally a gold-colored
metallic conductor, it becomes superconducting at very low temperatures, below 0.26 K.
For reasons of space, our discussion here will focus solely on S N .
4 4
+
S N S N
N S − N S + S S
−
N N N N
+ − −
S N S N S S
S N
4 4
N S N S
+
N
S 2 2
S S S
N N N
N N
N S S S n
polythiazyl
Figure 6.1 Selected sulfur nitride structures.
A modern synthesis of S N is the following (Maaninen, A.; Shvari, J.; Laitinen, R. S.;
4 4
Chivers, T. Inorg. Syn. 2002, 33, 196–199), where we have ignored the transannular
interactions shown above (i.e., the dotted lines) and represented S N simply as an
4 4
eight-membered ring:
Me 3 Si S SiMe 3
2 Me Si N N SiMe 3 + 2 SCl 2 + 2 SO 2 Cl 2
3
+
N S
N S −
+ S N (6.118)
S − N
8 Me 3 SiCl + 2 SO 2 + −
− N S
N S
+
S N
S N
+