Page 264 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 264
GROUP 16 ELEMENTS: THE CHALCOGENS
244
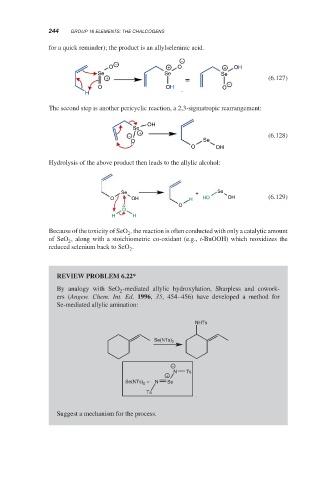
for a quick reminder); the product is an allylseleninic acid.
−
−
O + O + OH
Se Se Se
+ = (6.127)
O OH O −
H
The second step is another pericyclic reaction, a 2,3-sigmatropic rearrangement:
OH
Se
− + (6.128)
O Se
O OH
Hydrolysis of the above product then leads to the allylic alcohol:
Se + Se
O OH H HO OH (6.129)
O
O
H H
Because of the toxicity of SeO , the reaction is often conducted with only a catalytic amount
2
of SeO , along with a stoichiometric co-oxidant (e.g., t-BuOOH) which reoxidizes the
2
reduced selenium back to SeO .
2
REVIEW PROBLEM 6.22*
By analogy with SeO -mediated allylic hydroxylation, Sharpless and cowork-
2
ers (Angew. Chem. Int. Ed. 1996, 35, 454–456) have developed a method for
Se-mediated allylic amination:
NHTs
Se(NTs) 2
−
N Ts
+
Se(NTs) 2 = N Se
Ts
Suggest a mechanism for the process.