Page 269 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 269
6.17 HIGHER-VALENT TELLURIUM: A MECHANISTIC PUZZLE 249
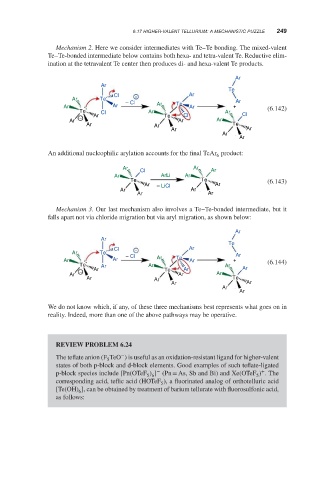
Mechanism 2. Here we consider intermediates with Te–Te bonding. The mixed-valent
Te–Te-bonded intermediate below contains both hexa- and tetra-valent Te. Reductive elim-
ination at the tetravalent Te center then produces di- and hexa-valent Te products.
Ar
Ar
Te
Cl − Ar
Ar Te
− Cl Ar Te Ar
Ar Ar Ar + (6.142)
Te Cl Ar Ar Cl
− Ar Te Cl
Ar Ar Ar
Ar Ar Te
Ar Ar
Ar
Ar
An additional nucleophilic arylation accounts for the final TeAr product:
6
Ar Ar
Cl Ar
Ar ArLi Ar
Te Te (6.143)
Ar – LiCl Ar
Ar Ar
Ar Ar
Mechanism 3. Our last mechanism also involves a Te–Te-bonded intermediate, but it
falls apart not via chloride migration but via aryl migration, as shown below:
Ar
Ar
Te
Cl − Ar
Ar Te Ar
Ar Ar − Cl Ar Te Ar + (6.144)
Te Ar Ar Ar
− Ar Te Ar Ar
Ar Ar Ar
Ar Ar Te
Ar Ar
Ar
Ar
We do not know which, if any, of these three mechanisms best represents what goes on in
reality. Indeed, more than one of the above pathways may be operative.
REVIEW PROBLEM 6.24
−
The teflate anion (F TeO ) is useful as an oxidation-resistant ligand for higher-valent
5
states of both p-block and d-block elements. Good examples of such teflate-ligated
− +
p-block species include [Pn(OTeF ) ] (Pn = As, Sb and Bi) and Xe(OTeF ) .The
5
5 6
corresponding acid, teflic acid (HOTeF ), a fluorinated analog of orthotelluric acid
5
[Te(OH) ], can be obtained by treatment of barium tellurate with fluorosulfonic acid,
6
as follows: