Page 270 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 270
GROUP 16 ELEMENTS: THE CHALCOGENS
250
OH − 2+ OH
HO O Ba − 2+
Te − + 5 O S
HO O F
O −
OH
Fluorosulfonic acid
2+ −
OH OH Ba O
F F − 2+ − 2+
Te + 4 S + O S −
F F O O OH O O
F − −
Teflic acid
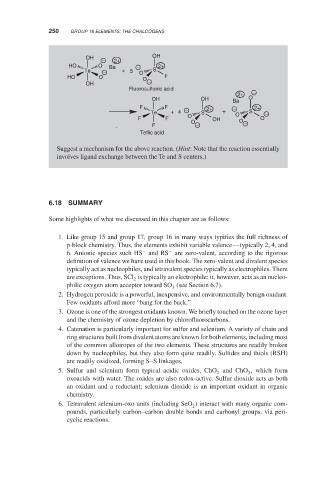
Suggest a mechanism for the above reaction. (Hint: Note that the reaction essentially
involves ligand exchange between the Te and S centers.)
6.18 SUMMARY
Some highlights of what we discussed in this chapter are as follows:
1. Like group 15 and group 17, group 16 in many ways typifies the full richness of
p-block chemistry. Thus, the elements exhibit variable valence—typically 2, 4, and
− −
6. Anionic species such HS and RS are zero-valent, according to the rigorous
definition of valence we have used in this book. The zero-valent and divalent species
typically act as nucleophiles, and tetravalent species typically as electrophiles. There
are exceptions. Thus, SCl is typically an electrophile; it, however, acts as an nucleo-
2
philic oxygen atom acceptor toward SO (see Section 6.7).
3
2. Hydrogen peroxide is a powerful, inexpensive, and environmentally benign oxidant.
Few oxidants afford more “bang for the buck.”
3. Ozone is one of the strongest oxidants known. We briefly touched on the ozone layer
and the chemistry of ozone depletion by chlorofluorocarbons.
4. Catenation is particularly important for sulfur and selenium. A variety of chain and
ring structures built from divalent atoms are known for both elements, including most
of the common allotropes of the two elements. These structures are readily broken
down by nucleophiles, but they also form quite readily. Sulfides and thiols (RSH)
are readily oxidized, forming S–S linkages.
5. Sulfur and selenium form typical acidic oxides, ChO and ChO , which form
3
2
oxoacids with water. The oxides are also redox-active. Sulfur dioxide acts as both
an oxidant and a reductant; selenium dioxide is an important oxidant in organic
chemistry.
6. Tetravalent selenium-oxo units (including SeO ) interact with many organic com-
2
pounds, particularly carbon–carbon double bonds and carbonyl groups, via peri-
cyclic reactions.