Page 240 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 240
GROUP 16 ELEMENTS: THE CHALCOGENS
220
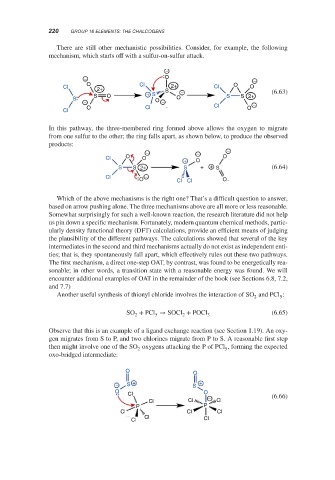
There are still other mechanistic possibilities. Consider, for example, the following
mechanism, which starts off with a sulfur-on-sulfur attack.
−
− O −
O Cl O
Cl 2+ Cl O
2+ S
S O + S − S S 2+ (6.63)
S O O
− − −
O Cl Cl O
Cl
In this pathway, the three-membered ring formed above allows the oxygen to migrate
from one sulfur to the other; the ring falls apart, as shown below, to produce the observed
products:
− − −
Cl O O + O O
S S 2+ S + + S (6.64)
Cl −
O Cl Cl O
Which of the above mechanisms is the right one? That’s a difficult question to answer,
based on arrow pushing alone. The three mechanisms above are all more or less reasonable.
Somewhat surprisingly for such a well-known reaction, the research literature did not help
us pin down a specific mechanism. Fortunately, modern quantum chemical methods, partic-
ularly density functional theory (DFT) calculations, provide an efficient means of judging
the plausibility of the different pathways. The calculations showed that several of the key
intermediates in the second and third mechanisms actually do not exist as independent enti-
ties; that is, they spontaneously fall apart, which effectively rules out these two pathways.
The first mechanism, a direct one-step OAT, by contrast, was found to be energetically rea-
sonable; in other words, a transition state with a reasonable energy was found. We will
encounter additional examples of OAT in the remainder of the book (see Sections 6.8, 7.2,
and 7.7)
Another useful synthesis of thionyl chloride involves the interaction of SO and PCl :
2
5
SO + PCl → SOCl + POCl 3 (6.65)
5
2
2
Observe that this is an example of a ligand exchange reaction (see Section 1.19). An oxy-
gen migrates from S to P, and two chlorines migrate from P to S. A reasonable first step
then might involve one of the SO oxygens attacking the P of PCl , forming the expected
2
5
oxo-bridged intermediate:
O O
− S + S +
O O
Cl (6.66)
Cl Cl − Cl
P P
Cl Cl Cl
Cl
Cl Cl