Page 236 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 236
GROUP 16 ELEMENTS: THE CHALCOGENS
216
REVIEW PROBLEM 6.5
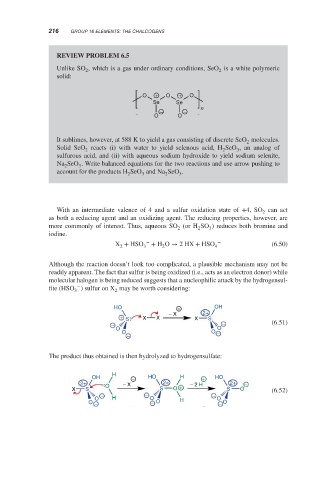
Unlike SO , which is a gas under ordinary conditions, SeO is a white polymeric
2 2
solid:
O + O + O
Se Se
− − n
O O
It sublimes, however, at 588 K to yield a gas consisting of discrete SeO molecules.
2
Solid SeO reacts (i) with water to yield selenous acid, H SeO , an analog of
3
2
2
sulfurous acid, and (ii) with aqueous sodium hydroxide to yield sodium selenite,
Na SeO . Write balanced equations for the two reactions and use arrow pushing to
2 3
account for the products H SeO and Na SeO .
2 3 2 3
With an intermediate valence of 4 and a sulfur oxidation state of +4, SO can act
2
as both a reducing agent and an oxidizing agent. The reducing properties, however, are
more commonly of interest. Thus, aqueous SO (or H SO ) reduces both bromine and
2
3
2
iodine.
− −
X + HSO + H O → 2HX + HSO (6.50)
2 3 2 4
Although the reaction doesn’t look too complicated, a plausible mechanism may not be
readily apparent. The fact that sulfur is being oxidized (i.e., acts as an electron donor) while
molecular halogen is being reduced suggests that a nucleophilic attack by the hydrogensul-
−
fite (HSO )sulfuronX may be worth considering:
3
2
HO − OH
+ S X X − X X 2+ S
− − (6.51)
O O
O O −
−
The product thus obtained is then hydrolyzed to hydrogensulfate:
H
OH − HO H + HO
2+ − X 2+ − 2 H 2+ −
X S O S O + S O (6.52)
− − −
O H O H O
O − − O − O