Page 232 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 232
GROUP 16 ELEMENTS: THE CHALCOGENS
212
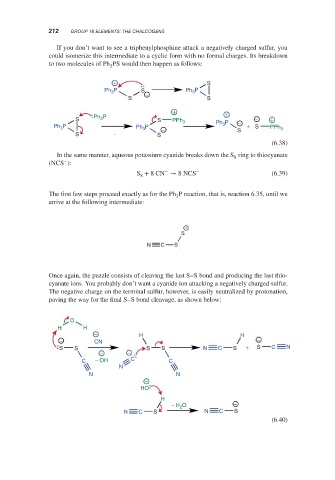
If you don’t want to see a triphenylphosphine attack a negatively charged sulfur, you
could isomerize this intermediate to a cyclic form with no formal charges. Its breakdown
to two molecules of Ph PS would then happen as follows:
3
+ S
Ph P S − Ph P
3
3
S S
+ +
Ph P
S 3 S PPh 3 Ph P − − +
Ph P Ph P − 3 S + S PPh 3
3
3
S S
(6.38)
In the same manner, aqueous potassium cyanide breaks down the S ring to thiocyanate
8
−
(NCS ):
− −
S + 8CN → 8NCS (6.39)
8
The first few steps proceed exactly as for the Ph P reaction, that is, reaction 6.35, until we
3
arrive at the following intermediate:
Once again, the puzzle consists of cleaving the last S–S bond and producing the last thio-
cyanate ions. You probably don’t want a cyanide ion attacking a negatively charged sulfur.
The negative charge on the terminal sulfur, however, is easily neutralized by protonation,
paving the way for the final S–S bond cleavage, as shown below:
O
H H
− H H
− CN −
S S S S N C S + S C N
− −
C − OH C C
N
N N
−
HO
H
− H O −
2
N C S N C S
(6.40)