Page 230 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 230
GROUP 16 ELEMENTS: THE CHALCOGENS
210
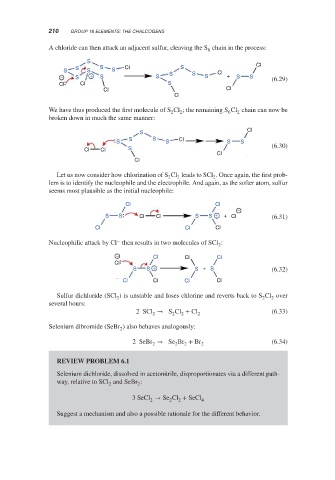
A chloride can then attack an adjacent sulfur, cleaving the S chain in the process:
8
S
S S Cl S Cl
S S S S Cl
− S + S S S S + S S (6.29)
Cl Cl S
Cl Cl
Cl
We have thus produced the first molecule of S Cl ; the remaining S Cl chain can now be
6
2
2
2
broken down in much the same manner:
Cl
S
S S Cl
S S S S
Cl Cl S (6.30)
Cl
Cl
Let us now consider how chlorination of S Cl leads to SCl . Once again, the first prob-
2 2 2
lem is to identify the nucleophile and the electrophile. And again, as the softer atom, sulfur
seems most plausible as the initial nucleophile:
Cl Cl
−
S S Cl Cl S S + + Cl (6.31)
Cl Cl Cl
−
Nucleophilic attack by Cl then results in two molecules of SCl :
2
− Cl Cl Cl
Cl
S S + S + S (6.32)
Cl Cl Cl Cl Cl
Sulfur dichloride (SCl ) is unstable and loses chlorine and reverts back to S Cl over
2 2 2
several hours:
2SCl → S Cl + Cl (6.33)
2 2 2 2
Selenium dibromide (SeBr ) also behaves analogously:
2
2 SeBr → Se Br + Br 2 (6.34)
2
2
2
REVIEW PROBLEM 6.1
Selenium dichloride, dissolved in acetonitrile, disproportionates via a different path-
way, relative to SCl and SeBr :
2
2
3 SeCl → Se Cl + SeCl 4
2
2
2
Suggest a mechanism and also a possible rationale for the different behavior.