Page 226 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 226
GROUP 16 ELEMENTS: THE CHALCOGENS
206
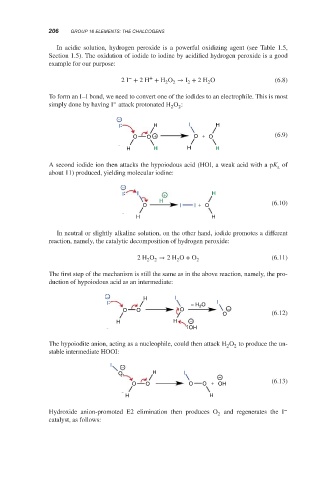
In acidic solution, hydrogen peroxide is a powerful oxidizing agent (see Table 1.5,
Section 1.5). The oxidation of iodide to iodine by acidified hydrogen peroxide is a good
example for our purpose:
− +
2I + 2H + H O → I + 2H O (6.8)
2 2 2 2
To form an I–I bond, we need to convert one of the iodides to an electrophile. This is most
−
simply done by having I attack protonated H O :
2
2
−
I H I H
O O + O + O (6.9)
H H H H
A second iodide ion then attacks the hypoiodous acid (HOI, a weak acid with a pK of
a
about 11) produced, yielding molecular iodine:
−
I I + H
H
O I I + O (6.10)
H H
In neutral or slightly alkaline solution, on the other hand, iodide promotes a different
reaction, namely, the catalytic decomposition of hydrogen peroxide:
2H O → 2H O + O (6.11)
2 2 2 2
The first step of the mechanism is still the same as in the above reaction, namely, the pro-
duction of hypoiodous acid as an intermediate:
− H I
I − H O I
2
O O O −
O (6.12)
H H −
OH
The hypoiodite anion, acting as a nucleophile, could then attack H O to produce the un-
2
2
stable intermediate HOOI:
I −
O H I
−
O O O O + OH (6.13)
H H
−
Hydroxide anion-promoted E2 elimination then produces O and regenerates the I
2
catalyst, as follows: