Page 225 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 225
6.2 THE DIVALENT STATE: HYDROGEN PEROXIDE 205
S S H S S + H −
H S S H S S + CI
S
S CI S (6.3)
S CI
CI
On leaving, the chloride ions pluck off protons from the cationic sulfurs, as shown below.
Another round of the same process then leads to the S product.
6
−
CI
S
S S H − HCI S S − HCI
H S S H S S S S S S
S + S
S
S S
CI CI
(6.4)
6.2 THE DIVALENT STATE: HYDROGEN PEROXIDE
Hydrogen peroxide is a relatively stable but reactive molecule. Dihydrogen trioxide or tri-
oxidane, HOOOH, is far more reactive, but has been generated in aqueous solution at low
temperature. The higher “oxidanes” H O and H O are also believed to exist but only as
5
2
2
4
fleeting intermediates. In other words, catenation is much more limited for oxygen than it
is for the other chalcogens.
With an oxidation state intermediate between those of water and O , hydrogen peroxide
2
exhibits a wide range of reactivity. Although it is best known as an oxidant, it reduces
stronger oxidants than itself while itself becoming oxidized to O . It can act as both a
2
nucleophile and an electrophile.
Hydrogen peroxide oxidizes sulfite to sulfate and organic sulfides to sulfoxides:
2− 2−
SO + H O → SO + H O (6.5)
3 2 2 4 2
PhSMe + H O → Ph-SO-Me + H O (6.6)
2
2
2
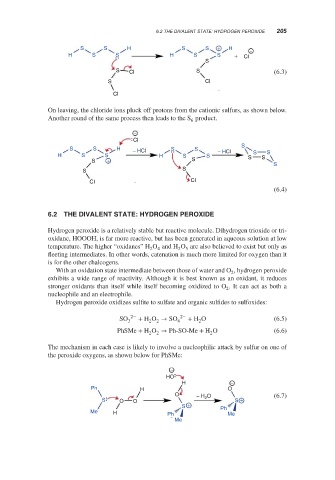
The mechanism in each case is likely to involve a nucleophilic attack by sulfur on one of
the peroxide oxygens, as shown below for PhSMe:
−
HO
H −
Ph H O
O − H O (6.7)
S O O 2 S +
S + Ph
Me H Ph Me
Me