Page 228 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 228
GROUP 16 ELEMENTS: THE CHALCOGENS
208
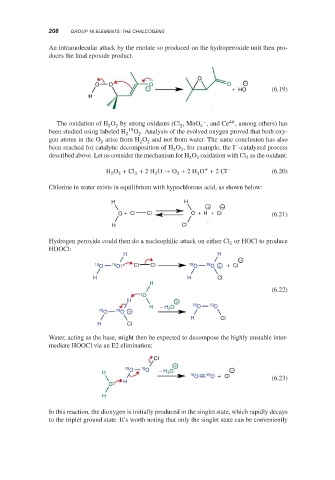
An intramolecular attack by the enolate so produced on the hydroperoxide unit then pro-
duces the final epoxide product.
O
O O O O −
− + HO (6.19)
H
4+
−
The oxidation of H O by strong oxidants (Cl ,MnO , and Ce , among others) has
2 2 2 4
been studied using labeled H 18 O . Analysis of the evolved oxygen proved that both oxy-
2 2
gen atoms in the O arise from H O and not from water. The same conclusion has also
2 2 2
−
been reached for catalytic decomposition of H O , for example, the I -catalyzed process
2 2
described above. Let us consider the mechanism for H O oxidation with Cl as the oxidant:
2
2
2
+ −
H O + Cl + 2H O → O + 2H O + 2Cl (6.20)
2
2
2
3
2
2
Chlorine in water exists in equilibrium with hypochlorous acid, as shown below:
H H
+ −
O + Cl Cl O + H + Cl (6.21)
H Cl
Hydrogen peroxide could then do a nucleophilic attack on either Cl or HOCl to produce
2
HOOCl:
H H
−
18 O 18 O Cl Cl 18 O 18 O + + Cl
H H Cl
H
(6.22)
O
H +
H − H 3 O 18 O 18 O
18 18 O +
O
H Cl
H Cl
Water, acting as the base, might then be expected to decompose the highly unstable inter-
mediate HOOCl via an E2 elimination:
Cl
+
18 18 −
H O O − H 3 O
18 O 18 O + Cl
H (6.23)
O
H
In this reaction, the dioxygen is initially produced in the singlet state, which rapidly decays
to the triplet ground state. It’s worth noting that only the singlet state can be conveniently