Page 227 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 227
6.2 THE DIVALENT STATE: HYDROGEN PEROXIDE 207
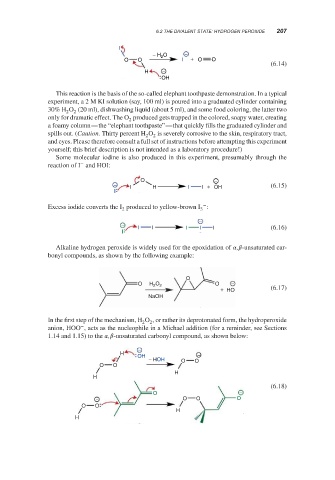
I
− H 2 O −
O O I + O O
(6.14)
H −
OH
This reaction is the basis of the so-called elephant toothpaste demonstration. In a typical
experiment, a 2 M KI solution (say, 100 ml) is poured into a graduated cylinder containing
30% H O (20 ml), dishwashing liquid (about 5 ml), and some food coloring, the latter two
2 2
only for dramatic effect. The O produced gets trapped in the colored, soapy water, creating
2
a foamy column—the “elephant toothpaste”—that quickly fills the graduated cylinder and
spills out. (Caution. Thirty percent H O is severely corrosive to the skin, respiratory tract,
2 2
and eyes. Please therefore consult a full set of instructions before attempting this experiment
yourself; this brief description is not intended as a laboratory procedure!)
Some molecular iodine is also produced in this experiment, presumably through the
−
reaction of I and HOI:
O −
− (6.15)
I H I I + OH
I
−
Excess iodide converts the I produced to yellow-brown I :
2 3
−
− I I I I I (6.16)
I
Alkaline hydrogen peroxide is widely used for the epoxidation of , -unsaturated car-
bonyl compounds, as shown by the following example:
O
O H 2 O 2 O −
+ HO (6.17)
NaOH
In the first step of the mechanism, H O , or rather its deprotonated form, the hydroperoxide
2
2
−
anion, HOO , acts as the nucleophile in a Michael addition (for a reminder, see Sections
1.14 and 1.15) to the , -unsaturated carbonyl compound, as shown below:
−
H −
OH
− HOH O O
O O
H
H
(6.18)
O −
− O O O
O O
H
H