Page 229 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 229
6.3 S Cl AND SCl
2 2 2 209
represented in a Lewis structure, that is, via a simple double-bonded structure (O=O). The
triplet ground state requires a molecular orbital description, which is presented in most
general and inorganic chemistry textbooks.
Let’s reflect for a moment on how the chemistry just described compares with the chem-
istry of the sulfanes described in the last section. Like the sulfane SH groups, the oxygens of
H O can act as nucleophiles. Like the sulfur atoms within polysulfide chains, the oxygens
2
2
in H O can also act as electrophiles. One distinct feature of H O chemistry is of course
2
2
2
2
its potential for oxidation to O . Sulfanes too are prone to oxidation but not to diatomic S ,
2
2
which is unstable, but rather to medium-sized S rings.
n
6.3 S Cl AND SCl 2
2
2
Passing chlorine through molten sulfur results in disulfur dichloride (S Cl ), a fuming
2
2
orange liquid. Further chlorination leads to sulfur dichloride (SCl ).
2
S + 4Cl → 4S Cl 2 (6.24)
2
8
2
S Cl + Cl → 2SCl 2 (6.25)
2
2
2
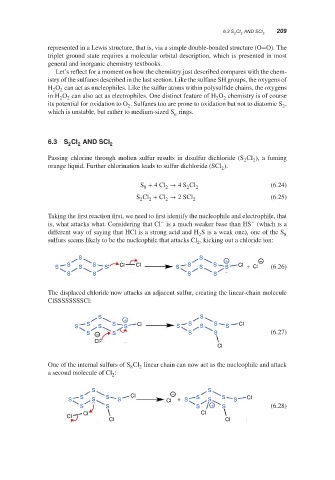
Taking the first reaction first, we need to first identify the nucleophile and electrophile, that
−
−
is, what attacks what. Considering that Cl is a much weaker base than HS (which is a
different way of saying that HCl is a strong acid and H S is a weak one), one of the S
2 8
sulfurs seems likely to be the nucleophile that attacks Cl , kicking out a chloride ion:
2
S S + −
S S S S S Cl Cl S S S S S Cl + Cl (6.26)
S S S S
The displaced chloride now attacks an adjacent sulfur, creating the linear-chain molecule
ClSSSSSSSSCl:
S + S
S S S S S Cl S S S S S Cl
S − S S S (6.27)
Cl
Cl
One of the internal sulfurs of S Cl linear chain can now act as the nucleophile and attack
2
8
a second molecule of Cl :
2
S S
S S Cl − S S Cl
S S S Cl + S S S
S S S + S (6.28)
Cl Cl
Cl
Cl Cl