Page 277 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 277
7.1 SOME NOTES ON ELEMENTAL HALOGENS 257
O O
+
N I N
O O
O O
Silver ions interact strongly with halide ions, and the stability of silver halides may drive
otherwise unlikely reactions such as the following:
I + AgClO → AgI + IClO 4 (7.7)
2
4
−
As an extremely weak base and a poor nucleophile, perchlorate (ClO ) would not normally
4
attack I , but silver ion coordination drives the process, as shown below:
2
−
O −
O
3+
− Cl + 3+ (7.8)
O O I I Ag − Cl I + AgI
O − O O
− O
−
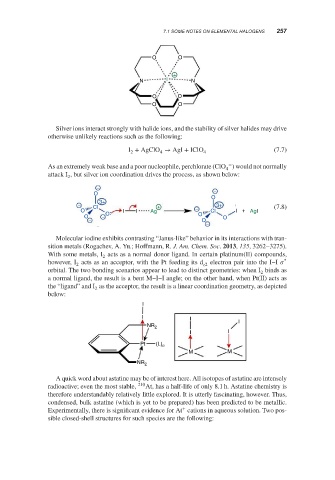
Molecular iodine exhibits contrasting “Janus-like” behavior in its interactions with tran-
sition metals (Rogachev, A. Yu.; Hoffmann, R. J. Am. Chem. Soc. 2013, 135, 3262–3275).
With some metals, I acts as a normal donor ligand. In certain platinum(II) compounds,
2
however, I acts as an acceptor, with the Pt feeding its d electron pair into the I–I *
2 z2
orbital. The two bonding scenarios appear to lead to distinct geometries: when I binds as
2
a normal ligand, the result is a bent M–I–I angle; on the other hand, when Pt(II) acts as
the “ligand” and I as the acceptor, the result is a linear coordination geometry, as depicted
2
below:
I
I I
NR 2 I
I I
Pt (L) n
M M
NR 2
A quick word about astatine may be of interest here. All isotopes of astatine are intensely
radioactive; even the most stable, 210 At, has a half-life of only 8.1 h. Astatine chemistry is
therefore understandably relatively little explored. It is utterly fascinating, however. Thus,
condensed, bulk astatine (which is yet to be prepared) has been predicted to be metallic.
+
Experimentally, there is significant evidence for At cations in aqueous solution. Two pos-
sible closed-shell structures for such species are the following: