Page 280 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 280
THE HALOGENS
260
−
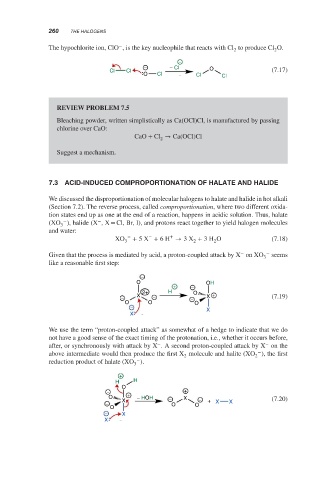
The hypochlorite ion, ClO , is the key nucleophile that reacts with Cl to produce Cl O.
2
2
−
− − Cl O
Cl Cl (7.17)
O Cl Cl Cl
REVIEW PROBLEM 7.5
Bleaching powder, written simplistically as Ca(OCl)Cl, is manufactured by passing
chlorine over CaO:
CaO + Cl → Ca(OCl)Cl
2
Suggest a mechanism.
7.3 ACID-INDUCED COMPROPORTIONATION OF HALATE AND HALIDE
We discussed the disproportionation of molecular halogens to halate and halide in hot alkali
(Section 7.2). The reverse process, called comproportionation, where two different oxida-
tion states end up as one at the end of a reaction, happens in acidic solution. Thus, halate
−
−
(XO ), halide (X ,X = Cl, Br, I), and protons react together to yield halogen molecules
3
and water:
− − +
XO + 5X + 6H → 3X + 3H O (7.18)
3 2 2
−
Given that the process is mediated by acid, a proton-coupled attack by X on XO − seems
3
like a reasonable first step:
−
O OH
+ −
2+ H O
− X − − X + (7.19)
O O O
−
X
X
We use the term “proton-coupled attack” as somewhat of a hedge to indicate that we do
not have a good sense of the exact timing of the protonation, i.e., whether it occurs before,
− −
after, or synchronously with attack by X . A second proton-coupled attack by X on the
−
above intermediate would then produce the first X molecule and halite (XO ), the first
2
2
−
reduction product of halate (XO ).
3
+
H H
O
− +
O + − HOH − X − (7.20)
− X O O + X X
O
− X
X