Page 279 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 279
7.2 ALKALI-INDUCED DISPROPORTIONATION OF MOLECULAR HALOGENS 259
− − −
3XO → XO 3 + 2X (7.12)
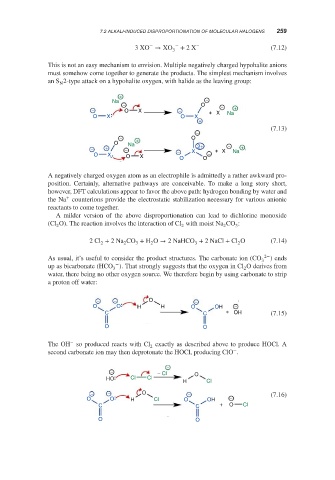
This is not an easy mechanism to envision. Multiple negatively charged hypohalite anions
must somehow come together to generate the products. The simplest mechanism involves
an S 2-type attack on a hypohalite oxygen, with halide as the leaving group:
N
+
Na −
− O −
− O X − + X Na +
O X O X
+
(7.13)
−
− + O
O Na
− + − − X 2+ + X − Na +
O X O X O O −
A negatively charged oxygen atom as an electrophile is admittedly a rather awkward pro-
position. Certainly, alternative pathways are conceivable. To make a long story short,
however, DFT calculations appear to favor the above path: hydrogen bonding by water and
+
the Na counterions provide the electrostatic stabilization necessary for various anionic
reactants to come together.
A milder version of the above disproportionation can lead to dichlorine monoxide
(Cl O). The reaction involves the interaction of Cl with moist Na CO :
2 2 2 3
2Cl + 2Na CO + H O → 2NaHCO + 2NaCl + Cl O (7.14)
2 2 3 2 3 2
As usual, it’s useful to consider the product structures. The carbonate ion (CO 3 2− ) ends
−
up as bicarbonate (HCO ). That strongly suggests that the oxygen in Cl O derives from
3 2
water, there being no other oxygen source. We therefore begin by using carbonate to strip
a proton off water:
− − O −
O O H H O OH −
C C + OH (7.15)
O O
−
The OH so produced reacts with Cl exactly as described above to produce HOCl. A
2
−
second carbonate ion may then deprotonate the HOCl, producing ClO .
−
− − Cl O
HO Cl Cl
H Cl
− − O − (7.16)
O O H Cl O OH −
C C + O Cl
O O