Page 283 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 283
7.4 HYPOFLUOROUS ACID, HOF 263
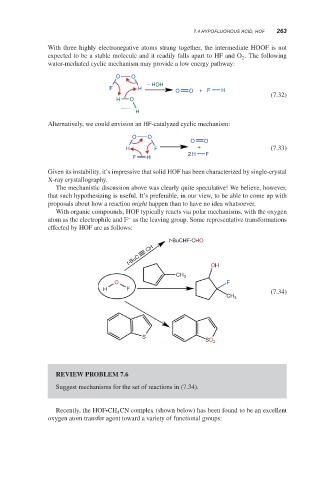
With three highly electronegative atoms strung together, the intermediate HOOF is not
expected to be a stable molecule and it readily falls apart to HF and O . The following
2
water-mediated cyclic mechanism may provide a low energy pathway:
O O
− HOH
F H
O O + F H
(7.32)
H O
H
Alternatively, we could envision an HF-catalyzed cyclic mechanism:
O O
O O
H F + (7.33)
2 H F
F H
Given its instability, it’s impressive that solid HOF has been characterized by single-crystal
X-ray crystallography.
The mechanistic discussion above was clearly quite speculative! We believe, however,
that such hypothesizing is useful. It’s preferable, in our view, to be able to come up with
proposals about how a reaction might happen than to have no idea whatsoever.
With organic compounds, HOF typically reacts via polar mechanisms, with the oxygen
−
atom as the electrophile and F as the leaving group. Some representative transformations
effected by HOF are as follows:
t-BuCHF-CHO
CH
t-BuC OH
CH 3
O F
H F (7.34)
CH 3
S
SO 2
REVIEW PROBLEM 7.6
Suggest mechanisms for the set of reactions in (7.34).
Recently, the HOF •CH CN complex (shown below) has been found to be an excellent
3
oxygen atom transfer agent toward a variety of functional groups: