Page 288 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 288
THE HALOGENS
268
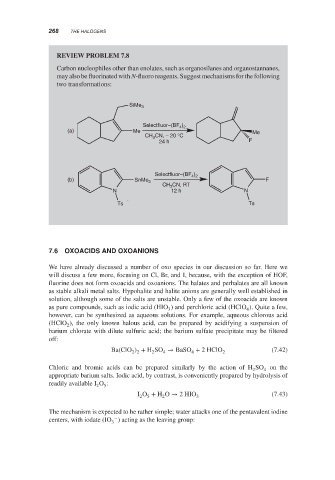
REVIEW PROBLEM 7.8
Carbon nucleophiles other than enolates, such as organosilanes and organostannanes,
may also be fluorinated with N-fluoro reagents. Suggest mechanisms for the following
two transformations:
SiMe 3
Selectfluor–(BF )
4 2
(a) Me Me
CH 3 CN, − 20 °C
24 h F
4
Selectfluor–(BF ) 2
(b) SnMe 3 F
CH CN, RT
3
N 12 h N
Ts Ts
7.6 OXOACIDS AND OXOANIONS
We have already discussed a number of oxo species in our discussion so far. Here we
will discuss a few more, focusing on Cl, Br, and I, because, with the exception of HOF,
fluorine does not form oxoacids and oxoanions. The halates and perhalates are all known
as stable alkali metal salts. Hypohalite and halite anions are generally well established in
solution, although some of the salts are unstable. Only a few of the oxoacids are known
as pure compounds, such as iodic acid (HIO ) and perchloric acid (HClO ). Quite a few,
3
4
however, can be synthesized as aqueous solutions. For example, aqueous chlorous acid
(HClO ), the only known halous acid, can be prepared by acidifying a suspension of
2
barium chlorate with dilute sulfuric acid; the barium sulfate precipitate may be filtered
off:
Ba(ClO ) + H SO → BaSO + 2HClO 2 (7.42)
2
4
2 2
4
Chloric and bromic acids can be prepared similarly by the action of H SO on the
2 4
appropriate barium salts. Iodic acid, by contrast, is conveniently prepared by hydrolysis of
readily available I O :
2 5
I O + H O → 2HIO (7.43)
2 5 2 3
The mechanism is expected to be rather simple; water attacks one of the pentavalent iodine
−
centers, with iodate (IO ) acting as the leaving group:
3