Page 292 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 292
THE HALOGENS
272
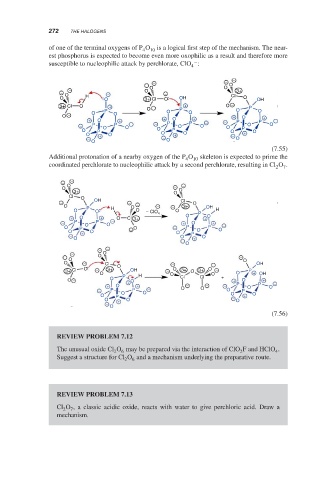
of one of the terminal oxygens of P O 10 is a logical first step of the mechanism. The near-
4
est phosphorus is expected to become even more oxophilic as a result and therefore more
−
susceptible to nucleophilic attack by perchlorate, ClO :
4
− O − O − O −
− − − O − 3+
O O H O 3+ Cl O OH Cl O OH
3+ Cl O + O − + O − P
P O P O O O
O − O O + O +
+ O + + O + − − P P −
− P O P O − − P O P O O O O
O P O P O P O
O + O O + O − +
− − O O
O
(7.55)
Additional protonation of a nearby oxygen of the P O skeleton is expected to prime the
4 10
coordinated perchlorate to nucleophilic attack by a second perchlorate, resulting in Cl O .
7
2
− −
O −
O − O
3+ O
Cl O
− OH − Cl O
O O − − − 3+ OH
O P O H O − ClO 4 O P H
O O
+ O + O Cl 3+ +
− P O P O − + O +
O P O − P P −
O + O − O P O O
− O O
O +
−
O
− −
− O
− O O −
O − Cl O − − O OH
3+ Cl O − 3+ OH − O 3+ O 3+ O − P
O H O O O + OH
O − O P O Cl Cl + + O +
+ P P −
+ O + − − − O O
− P O P O − O O O P
O P O + O
O + O − O
−
O
(7.56)
REVIEW PROBLEM 7.12
The unusual oxide Cl O may be prepared via the interaction of ClO F and HClO .
4
2
2
6
Suggest a structure for Cl O and a mechanism underlying the preparative route.
6
2
REVIEW PROBLEM 7.13
Cl O , a classic acidic oxide, reacts with water to give perchloric acid. Draw a
2
7
mechanism.