Page 289 - Arrow Pushing in Inorganic Chemistry A Logical Approach to the Chemistry of the Main Group Elements
P. 289
7.6 OXOACIDS AND OXOANIONS 269
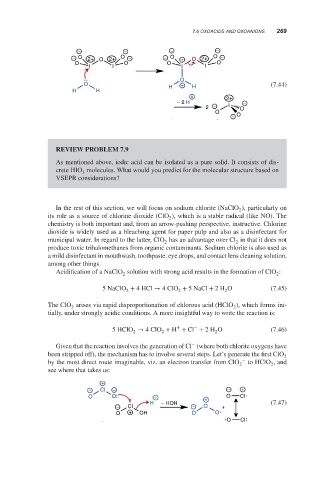
− − − −
− O 2+ O 2+ O − − O + O 2+ O −
O O O O
I I I I
O
O H + H (7.44)
H H
+ 2+
− 2 H I −
2 − O
O
− O
REVIEW PROBLEM 7.9
As mentioned above, iodic acid can be isolated as a pure solid. It consists of dis-
crete HIO molecules. What would you predict for the molecular structure based on
3
VSEPR considerations?
In the rest of this section, we will focus on sodium chlorite (NaClO ), particularly on
2
its role as a source of chlorine dioxide (ClO ), which is a stable radical (like NO). The
2
chemistry is both important and, from an arrow-pushing perspective, instructive. Chlorine
dioxide is widely used as a bleaching agent for paper pulp and also as a disinfectant for
municipal water. In regard to the latter, ClO has an advantage over Cl in that it does not
2
2
produce toxic trihalomethanes from organic contaminants. Sodium chlorite is also used as
a mild disinfectant in mouthwash, toothpaste, eye drops, and contact lens cleaning solution,
among other things.
Acidification of a NaClO solution with strong acid results in the formation of ClO :
2
2
5NaClO + 4HCl → 4ClO + 5NaCl + 2H O (7.45)
2
2
2
The ClO arises via rapid disproportionation of chlorous acid (HClO ), which forms ini-
2
2
tially, under strongly acidic conditions. A more insightful way to write the reaction is:
+ −
5HClO → 4ClO + H + Cl + 2H O (7.46)
2 2 2
−
Given that the reaction involves the generation of Cl (where both chlorite oxygens have
been stripped off), the mechanism has to involve several steps. Let’s generate the first ClO 2
by the most direct route imaginable, viz. an electron transfer from ClO − to HClO , and
2 2
see where that takes us:
+
− Cl − − +
O O + + O Cl
H − HOH (7.47)
− Cl − Cl +
O + OH O O
O Cl